Validation of a biotherapeutic immunoaffinity-LC–MS/MS assay in monkey serum: ‘plug-and-play’ across seven molecules
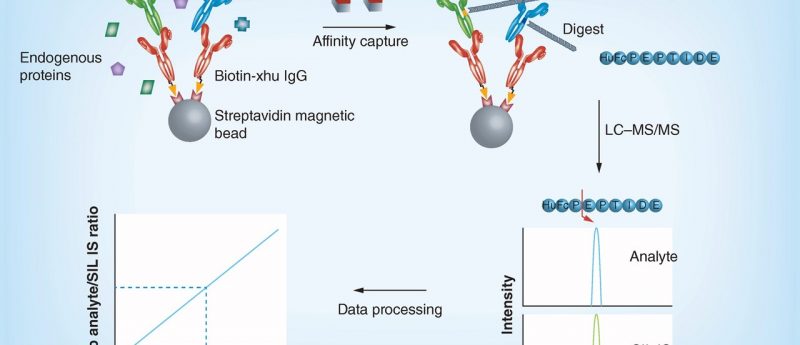
ABSTRACT Background: Biotherapeutics development requires validated assays in biological matrices for pharmacokinetic assessment. Historically, ligand-binding assays have been the predominant platform available. Recently, alternative hybrid methods, combining ligand-binding analyte enrichment with LC–MS detection have emerged. Methodology & results: The validation of an immunoaffinity (IA)-LC–MS/MS method to quantify a monoclonal antibody biotherapeutic in cynomolgus monkey serum is described. This method includes immunoaffinity capture of the antibody in serum, followed by enzymatic digestion and detection of a framework peptide. Using similar method conditions, six additional biotherapeutic assays were readily validated in different nonhuman mammalian species, including mouse, rat and monkey. Conclusion: The...






