Bioanalytical outsourcing: the two sides of the medal

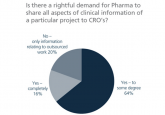
“In regulated bioanalysis, the reduction of in-house laboratory capacity in pharmaceutical companies is likely to continue, increasing the demand for qualified outsourcing staff and CRO capacity.” Recent issues and challenges in the pharmaceutical industry, such as cost and efficiency of drug development, the present and future role of outsourcing and the financial pressure lasting on contract research organizations (CROs) from a CRO perspective have been summarized in two editorials [1,2]. This commentary presents a more personal perspective of outsourcing, by an author who spent most of his career in regulated and nonregulated bioanalytics in major pharmaceutical companies, as well as...