Establishment of a Bioanalytical Strategy for a new Drug Candidate posing extreme Instability Issues
Andreas Gloge, Principle Scientist at F. Hoffmann-La Roche (Basel, Switzerland)
Andreas Gloge is a Bioanalytical Manager in R&D Bioanalytics at F. Hoffmann-La Roche (Basel, Switzerland). After receiving his PhD in biochemistry from the University of Karlsruhe (Germany), he completed a post-doctoral fellowship at Novartis/Syngenta (Basel, Switzerland) and then joined Roche (Basel, Switzerland) as a Bioanalytical Laboratory Head. He has nearly 20 years of experience in the field of regulated bioanalysis supporting preclinical and clinical bioanalysis for small molecules.

1. Please can you explain about your role at Roche?
I am a Principle Scientist working in the Small Molecules Bioanalytics R&D department at F. Hoffmann-La Roche. In my role of a Bioanalytical Manager, I support activities performed in-house and at our external partners overseeing the entire field from discovery to post-marketing. I am also responsible for maintaining the internal laboratory information management system.
2. How does it feel to win the 12th WRIB Poster Award?
It was a great honor and a privilege to be considered for this poster award. Considering that there were plenty of excellent posters presented at the WRIB, I value the award even higher.
3. Tell us a little about the work on which the poster was based?
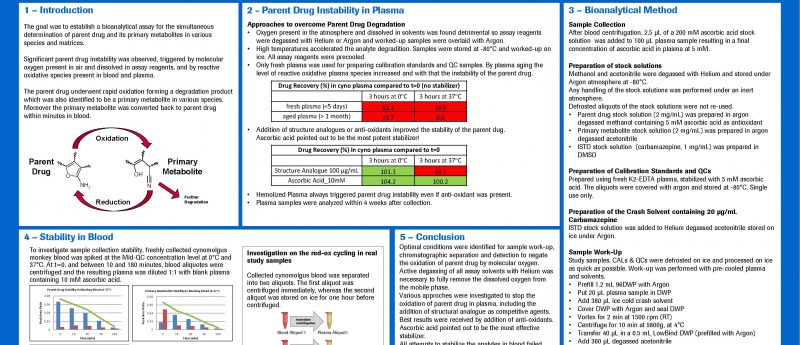
The poster gives an overview about investigations performed to overcome instability issues of a small molecule drug and its primary metabolite.
The parent drug underwent rapid oxidation, forming a degradation product which was also identified to be a primary metabolite in various species. The oxidation was triggered by molecular oxygen present in air and dissolved in assay reagents and by reactive oxidative species present in blood and plasma. Unconventional measures were implemented to avoid parent drug oxidation during sample storage, sample preparation, analyte separation and detection, like the active degassing of all assay reagents with helium and using inert atmosphere. Ascorbic acid was used as anti-oxidant to avoid degradation in plasma.
However all attempts to prevent parent drug degradation in blood failed. Moreover, in blood the primary metabolite was converted back to parent drug within minutes. Although we were not able to stop the red-ox cycling between parent drug and metabolite in blood, we have proven that in monkey blood stored over ice, the sum of both analytes remained unaffected over time and that the analyte concentrations reached a steady state after approximately 90 minutes. Once this equilibrium was reached no further degradation could be observed.
4. What were the key conclusions from your research?
We asked ourselves whether it was possible to develop a drug without being able to determine its concentration in the body. Due to the difficulties faced in stabilizing the parent drug and its primary metabolite in blood, we discovered a solution which was to report the sum of both. Because the primary metabolite is converted back to the parent drug, their sum could be considered analogous to the sum of a drug and it’s pro-drug. In the end, the project was not progressed because of non-BA related reasons and therefore this approach never faced regulatory scrutiny. Consequently we do not know whether this approach would have been considered acceptable to the regulatory authorities. This is an excellent example of a seemingly insurmountable bioanalytical challenge which was eventually overcome, even if untrodden paths full of obstacles had to be followed. However there is no better reason to expend every effort to support our project teams than that of patients awaiting more effective medicines.
5. What are you looking forward to working on over the next year?
I hope to get the chance to work on further challenging projects according to the theme:
“Problems are not stop signs, they are guidelines” – Robert H. Schuller.
Acknowledgements
I would like to thank Simona Maria Baratte and Rossella Pisano from Accelera S.r.l. for the valuable contribution to that work, especially for the evaluation for the blood stabilities.