Thermo Fisher’s oncomine precision assay granted breakthrough device designation
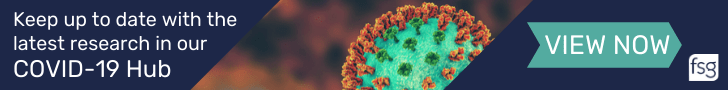
Thermo Fisher Scientific (Dartford, UK) have announced the US FDA has granted Breakthrough Device Designation for their Oncomine Precision Assay for identifying low-grade glioma patients with isocitrate dehydrogenase (IDH) 1 and 2 mutations who may be eligible for vorasidenib (AG-881), a first-in-class IDH1/IDH2 inhibitor. The assay, first introduced in November 2019, is designed to run on Thermo Fisher’s Ion Torrent Genexus System-the first fully automated next-generation sequencing platform with a specimen-to-report workflow that is capable of delivering comprehensive genetic profiling results within a day.
Thermo Fisher recently announced they had expanded their affiliation with Agios Pharmaceuticals (MA, USA) to co-develop the companion diagnostic for vorasidenib. Vorasidenib is an oral, brain-penetrant, dual inhibitor of mutant IDH1 and IDH2 enzymes currently under evaluation in the Phase III INDIGO study for IDH mutant low-grade glioma. In the future, Thermo Fisher is aiming to receive premarket approval for the Oncomine Precision Assay as a companion diagnostic for multiple therapies, as well as approval for liquid biopsy tumor profiling in lung cancer and solid tissue tumor profiling in multiple cancer types.
Alain Mita, co-director of the Experimental Therapeutics Program at Cedars-Sinai Medical Center (CA, USA), explained: “Access to timely, comprehensive genomic profiling data that supports well-informed treatment decisions can be challenging under the current cancer-testing paradigm. The possibility of having multi-biomarker profiling that is generated onsite and available in about a day is game-changing for the manner and speed in which oncologists are able to determine and prescribe the most appropriate treatment for their patients.”
The purpose of the Breakthrough Device Program by the FDA is to provide patients and health care providers with timely access to devices crucial for increasing the speed of their assessment and diagnoses, without compromising the agency’s statutory standards. Once the Oncomine Precision Assay has been cleared by this program, it will useful for maximizing detection of guideline-recommended biomarkers, such as EGFR, KRAS, BRAF, HER2 and others.
When combined with the Ion Torrent Genexus System, next-generation sequencing results are capable of being generated within the same timeframe as an existing single-gene test. In time to come, these features may also allow molecular pathologists to analyze next-generation sequencing information in parallel with first-line testing modalities, such as immunohistochemistry. Since its launch in 2019, the sequencer has accelerated a broad range of areas including (among others), oncology and infectious diseases. As a research only solution, the integrated sequencer has also been enabled to analyze SARS-CoV-2 samples to support epidemiology or contact tracing studies.
Garret Hampton (Thermo Fisher Scientific) concluded: “Breakthrough designation for the companion diagnostic is a big step forward in our endeavor to ensure that more clinicians can have quicker access to comprehensive genomic information. Receiving this insight at the speed that the Genexus System enables can help expedite patient therapy selection, which is a critical need in the clinic today.”