Validation of biomarkers survey infographic

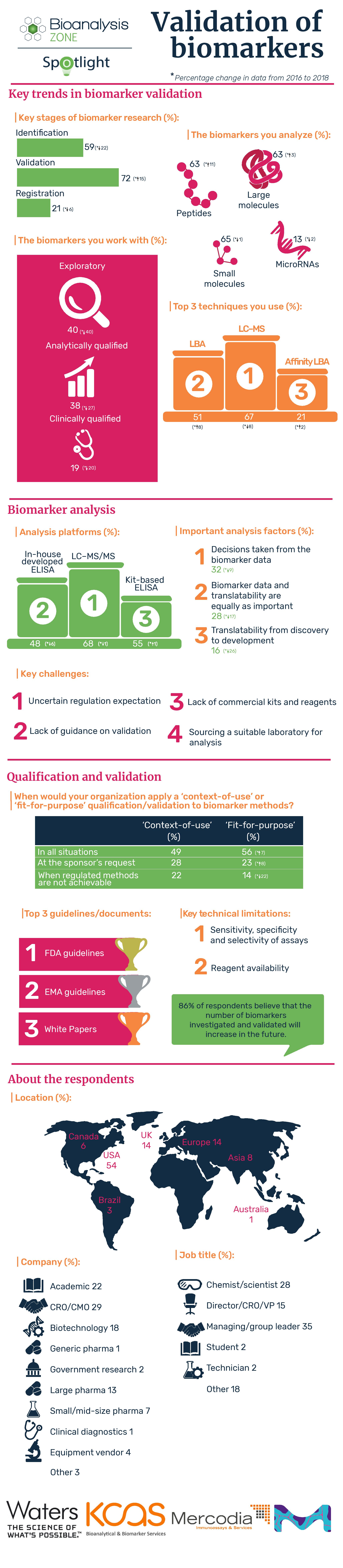
As part of our Spotlight on the validation of biomarkers, we conducted a survey and compared the results to previous data. Find out more about the key trends, challenges and future potential for biomarker R&D in this informative infographic.
DON’T MISS the panel discussion, where our experts will be discussing the results of the survey and more about biomarker R&D.