Novel multiplexed serologic test could diagnose Lyme disease in 15 minutes

Researchers based at Columbia University (SC, USA) have reported the development of a single multiplexed assay that can detect Lyme disease. The novel technique performs similarly to the current standard two-tiered diagnostic technique but in a much shorter time of 15 minutes.
In the USA approximately 300,000 people are diagnosed annually with Lyme disease, transmitted by infected Ixodes ticks. Without intervention, Lyme disease can cause cardiac, neurological and rheumatologic complications. The current diagnosis procedure utilizes a combination of ELISA and western blot assays to detect antibodies against the bacterium, taking several hours for experienced lab personnel to perform and interpret.
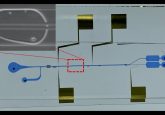
In the article, published in the Journal of Clinical Microbiology, researchers describe the development of a single multiplexed assay that utilizes a microfluidic platform. The assay is capable of detecting Lyme disease with success rates comparable to the currently used two-tiered approach in 15 minutes compared with several hours.
The team evaluated 142 samples from a wide demographic of patients with various stages of Lyme disease, first screening for a known set of Lyme disease diagnostic biomarkers. After having identified the top three biomarkers, the newly developed microfluidic platform, termed the mChip-Ld, was used to test the samples, along with the standard enzyme immunoassays.
The data demonstrated that the multiplexed set of biomarkers retained high levels of selectivity and displayed increased levels of sensitivity, with an improved ability to detect signs of infection in early-stage samples. The scientists speculated that the improved ability to detect early signs of disease infection may be due to the ability to detect antibodies that peak in the first weeks following infection.
The newly developed mChip-Ld platform achieved promising results when performing the diagnostic test and researchers hope the single multiplexed assay has the potential to be developed to a point-of-care test for Lyme disease. Siddarth Armumugam, co-author (Columbia University) explained: “While the assay will require more refinement and testing before it can be approved for widespread use as a test for Lyme disease, our results are very exciting.”
Samuel Sia, Professor of Biomedical Engineering at Columbia University, commented: “Our findings are the first to demonstrate that Lyme disease diagnosis can be carried out in a microfluidic format that can provide rapid quantitative results. This means that our test could easily be used directly in a doctor’s office, obviating having to send the samples out to a laboratory that needs at least a couple of hours, if not days, to get test results.”
Sources: Arumugam S, Nayak S, Williams T et al. A multiplexed serologic test for diagnosis of Lyme disease for point-of-care use. J. Clin. Microbiol. doi:10.1128/JCM.01142-19 (2019)(Epub ahead of print); https://engineering.columbia.edu/press-releases/sam-sia-lyme-disease-test