How blockchain and AI are transforming data integrity in bioanalysis
In this expert opinion piece, Seema Rajaram explains how blockchain and artificial intelligence (AI) are transforming bioanalytical data management in pharmaceutical development by providing immutable, tamper-evident record-keeping through blockchain technology and enhanced pattern recognition and error detection through AI systems. The integration of these technologies significantly improves data integrity, reduces compliance risks, and streamlines regulatory processes while meeting strict FDA and international pharmaceutical standards. Read on to find out how you can leverage blockchain technology to enhance data integrity in bioanalysis.
Bioanalysis plays a pivotal role in drug development, serving as the foundation for quantifying drug concentrations in biological matrices, elucidating pharmacokinetic and pharmacodynamic profiles, and ensuring therapeutic efficacy and safety. As the volume and complexity of bioanalytical data continue to grow, maintaining data accuracy, security and regulatory compliance has become increasingly challenging.
Emerging technologies such as blockchain and AI offer transformative potential in addressing these challenges. Far from being mere technological trends, these innovations provide robust frameworks for enhancing data integrity, optimizing operational workflows and ensuring adherence to global regulatory standards, including the US Food and Drug Administration’s (MD, USA) 21 CFR Part 11 and the International Council for Harmonization’s (Geneva, Switzerland) ICH E6 (R2) guidelines.
The importance of data integrity in bioanalysis
Data integrity is a cornerstone of regulated bioanalytical practices, underpinning the credibility of scientific outcomes and the safety of pharmaceutical interventions. Regulatory bodies across the globe strictly adhere to comprehensive data governance frameworks, most notably ALCOA+ principles, which define the essential attributes of trustworthy data:
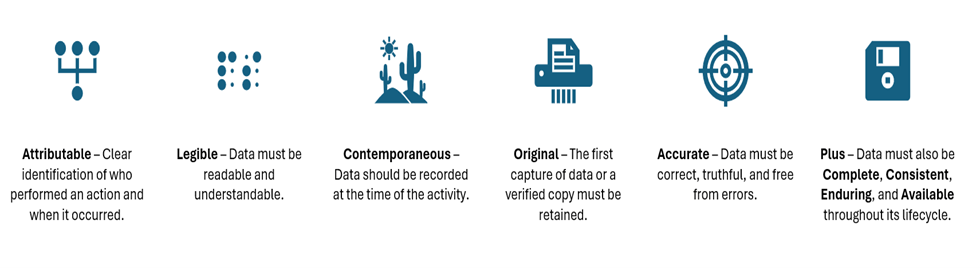
Non-compliance with these principles can have serious implications, including regulatory citations, audit failures, delays in product approval, and in severe cases, the distribution of unsafe pharmaceutical products. Therefore, maintaining rigorous data integrity is not only a regulatory obligation but a fundamental ethical responsibility in the development of safe and effective therapeutics.
Leveraging blockchain technology to enhance data integrity
Blockchain technology offers a decentralized, immutable digital ledger system that records transactions and data entries in a secure, transparent and tamper-evident manner. Once a record is added to the blockchain, it cannot be altered retroactively without consensus from the network, thereby ensuring the authenticity and traceability of data throughout its lifecycle.
Applied example in bioanalytical context
Consider a scenario in which a laboratory technician logs a biological sample collection into a blockchain-based system. This entry is automatically timestamped and cryptographically secured. Any subsequent attempt to modify the records such as altering the collection time or sample ID would be immediately detected and flagged by the system, preserving the integrity of the original data.
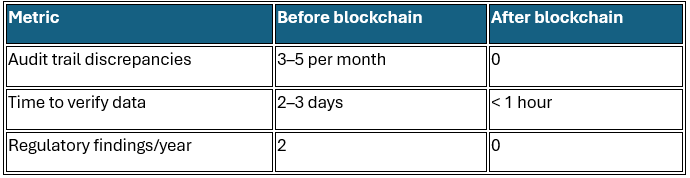
Impact metrics: Pre- and post-blockchain implementation
Key advantages of blockchain in bioanalysis
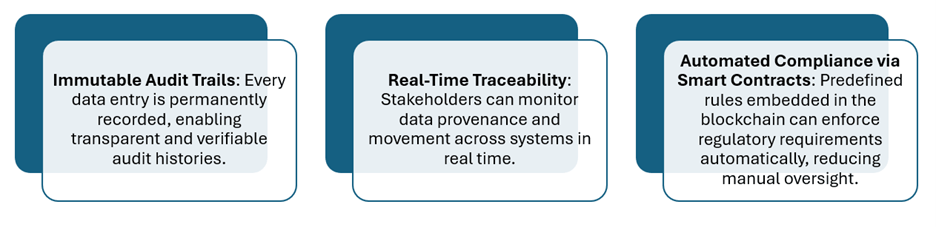
These advancements highlight the transformative potential of blockchain technology in reinforcing data governance, enhancing regulatory compliance and improving the overall reliability of bioanalytical processes within pharmaceutical development. A notable example is the MediLedger Project, which illustrates the practical application of blockchain in securing pharmaceutical supply chains and ensuring data integrity across complex regulatory environments.
Enhancing bioanalytical efficiency and compliance through AI
AI is increasingly recognized as a transformative tool in bioanalytical laboratories, offering the ability to process vast datasets rapidly and identify complex patterns that may elude manual review. Its integration into regulated environments supports both operational efficiency and regulatory compliance.
AI’s key applications in bioanalysis

Case study example
In a recent bioanalytical study involving 1,200 samples, an AI system identified 18 outlier results that would have otherwise required extensive manual review. This intervention not only saved several days of labor but also mitigated potential compliance risks by ensuring timely data validation.
Quantitative impact of AI integration
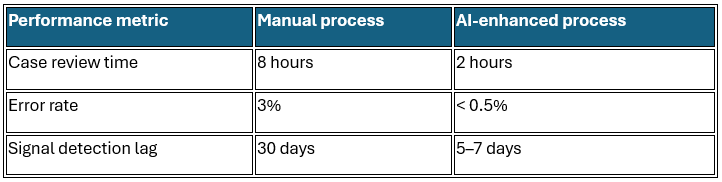
These metrics underscore AI’s potential to streamline bioanalytical workflows, reduce human error, and support proactive quality assurance in pharmaceutical development.
Integration framework for AI and blockchain in bioanalytical systems
The integration of AI and blockchain technologies within bioanalytical workflows offers a robust framework for enhancing data integrity, operational efficiency and regulatory compliance.
A hybrid architecture typically comprises the following layers:

Illustrative workflow
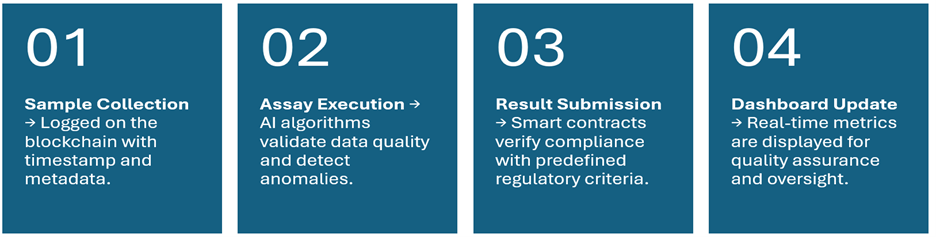
Regulatory considerations for AI and blockchain integration in bioanalysis
Regulatory authorities are increasingly supportive of digital innovation in pharmaceutical research, provided such technologies comply with established validation and documentation standards. Initiatives such as the FDA’s Digital Health Innovation Action Plan and the EMA’s Big Data Task Force reflect a growing institutional endorsement of advanced tools that enhance data integrity, transparency and operational efficiency.
- To be deployed in regulated bioanalytical environments, AI and blockchain systems must:
- Undergo GxP validation: Demonstrate reliability, reproducibility and suitability for intended use in accordance with good practice guidelines.
- Comply with 21 CFR Part 11: Ensure electronic records and signatures meet FDA standards for authenticity, integrity and confidentiality.
- Support audit-ready documentation: Generate complete, traceable records that facilitate regulatory inspections and quality assurance.
Final thoughts
Blockchain and AI represent transformative technologies in the pursuit of robust bioanalytical data integrity. Their integration into research and development quality assurance frameworks offers significant advantages, including enhanced transparency, reduced error rates and streamlined regulatory compliance. As the pharmaceutical industry continues to embrace digital transformation, these technologies are poised to become foundational components of modern bioanalytical practice.
Suggested reference articles, journals, case studies and examples for further reading
Below is a curated list of peer-reviewed articles, industry case studies, and authoritative sources that support the integration of blockchain and AI in bioanalysis, particularly in the context of data integrity, regulatory compliance and digital transformation:
- Blockchain for Data Integrity in Pharmaceutical Quality Assurance
- Block Chain-Enabled Data Analytics for Ensuring Data Integrity and Trust in AI Systems
- The Integration of Artificial Intelligence in Bioanalysis and Its Potential to Create a New Era of Diagnostics
- The Integration of AI and Blockchain in Healthcare: Ensuring Data Security and Integrity
- Blockchain, Artificial Intelligence, and Healthcare: The Tripod of Future – A Narrative Review
- Implementing AI-Driven Digital Transformation in Bioanalysis
- Blockchain and Pharmaceuticals: A Bibliometric and Systematic Literature Review
- Overcoming Bioanalysis Challenges With AI
- MediLedger Project – Blockchain for Drug Verification and Supply Chain Integrity
- Embleema – Blockchain for Clinical Trials and Patient Data
- IBM Food Trust – Blockchain for Pharma Supply Chain
- Medifakt – Blockchain + IoT + AI for Healthcare
- Chronicled – Blockchain for Regulatory Compliance
Disclaimer: the opinions expressed are solely that of the authors and do not express the views or opinions of their employers, Bioanalysis Zone or Taylor & Francis Group.
About the author:
 Seema Rajaram is a global test and validation leader specializing in digital transformation and artificial intelligence within the pharmaceutical industry. With over 18 years of experience in IT, she oversees complex global initiatives, leveraging AI and data science to enhance drug discovery and regulatory compliance processes. Her work focuses on developing and implementing advanced testing frameworks for AI-driven systems, ensuring data integrity, reliability and operational efficiency. Rajaram also plays a crucial role in risk management and security testing, protecting sensitive patient data and intellectual property in the digital pharmaceutical landscape. You can connect with Rajaram on LinkedIn.
Seema Rajaram is a global test and validation leader specializing in digital transformation and artificial intelligence within the pharmaceutical industry. With over 18 years of experience in IT, she oversees complex global initiatives, leveraging AI and data science to enhance drug discovery and regulatory compliance processes. Her work focuses on developing and implementing advanced testing frameworks for AI-driven systems, ensuring data integrity, reliability and operational efficiency. Rajaram also plays a crucial role in risk management and security testing, protecting sensitive patient data and intellectual property in the digital pharmaceutical landscape. You can connect with Rajaram on LinkedIn.
Our expert opinion collection provides you with in-depth articles written by authors from across the field of bioanalysis. Our expert opinions are perfect for those wanting a comprehensive, written review of a topic or looking for perspective pieces from our regular contributors.
See an article that catches your eye? Read any of our Expert Opinions for free.