Novel imaging method tracks skin drug movement in just 10 minutes
A new, rapid imaging approach precisely tracks how topical and transdermal medications penetrate skin layers without using dyes or labels.
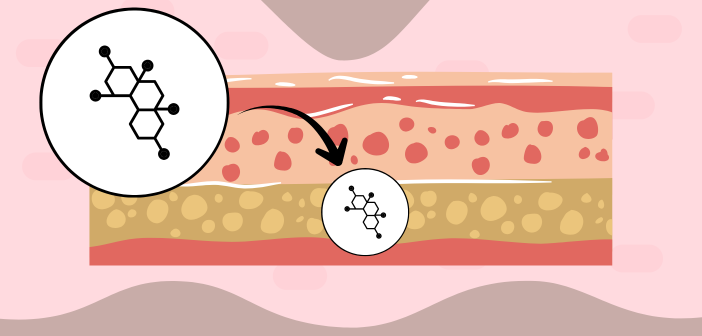
Researchers at the Hebrew University of Jerusalem’s School of Pharmacy (Jerusalem, Israel) have developed a new imaging technique that could offer faster, safer and more accurate design of topical and transdermal treatments. The method, which combines mass spectrometry imaging (MSI) with automated analysis, maps how medications move through the skin in just 10 minutes, without the need for dyes or labels.
Accurately tracking how far and fast drugs penetrate the skin requires extraordinary precision and is crucial for developing safe formulations, minimizing side effects and ensuring that active ingredients reach intended target layers. Some treatments, such as nicotine or hormone patches, must penetrate deeply enough to reach the bloodstream, while others, like antifungal or acne creams, need to remain near the surface and target a specific sublayer of skin.
You may also be interested in:
- In the Zone: targeted MS imaging for molecular spatial information
- Waters upgrade Xevo MRT performance: who doesn’t want sharper, faster imaging?
- Advanced MS imaging for targeted spatial molecular analysis
Traditionally, determining how far a compound travels has involved fluorescent tagging or radioactive tracers, which can alter the drug’s behavior or provide incomplete data. These processes are also time-consuming, often taking several days and requiring laborious image interpretation.
Led by Katy Margulis, the team from Hebrew University has addressed these challenges by integrating MSI with a computational tool that automatically segments skin tissue into layers and visualizes where active ingredients accumulate. The system also supports kinetic studies, allowing researchers to observe how quickly and deeply a drug moves through the skin, an advance that could accelerate the development of time-sensitive or personalized treatments.
“This approach gives us a clear, label-free snapshot of where a drug actually goes once applied to the skin. It allows researchers and developers to optimize delivery systems quickly and with much greater accuracy,” commented Margulis.
Tested on the antifungal medication terbinafine, the method revealed distinct distribution patterns across three nanoscale dermal delivery systems, demonstrating that different delivery systems led to markedly different permeation depths, with transethosomes (advanced vesicle delivery systems) achieving the deepest reach. Beyond antifungal therapies, this innovation could transform topical applications, such as corticosteroids and retinoids, by allowing real-time, label-free mapping of drug behavior in the skin.
“This tool allows for smarter, safer, and more efficient formulation testing,” explained Margulis. “Ultimately, it helps bring better products to patients faster.”