Method validation of quantitation IL-17A using the Quanterix Simoa™ platform
In this poster, the authors discuss the use of a Simoa Human IL-17A assay, which is a three-step digital immunoassay for the quantitation of total IL-17A in human serum. IL-17A plays an important role in the development of chronic inflammation and potentially in hemostatic disorders. The method used was validated using the Quanterix Simoa HD-1 Analyzer.
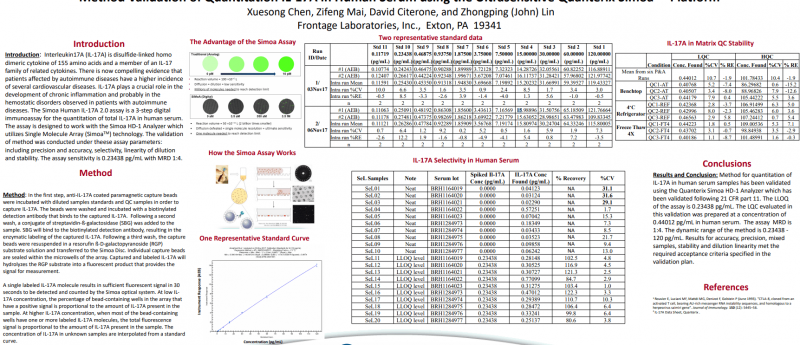
Introduction: Interleukin17A (IL-17A) is disulfide-linked homo dimeric cytokine of 155 amino acids and a member of an IL-17 family of related cytokines. There is now compelling evidence that patients affected by autoimmune diseases have a higher incidence of several cardiovascular diseases. IL-17A plays a crucial role in the development of chronic inflammation and probably in the hemostatic disorders observed in patients with autoimmune diseases. The Simoa Human IL-17A 2.0 assay is a 3-step digital immunoassay for the quantitation of total IL-17A in human serum. The assay is designed to work with the SimoaHD-1 Analyzer which utilizes Single Molecule Array (SimoaTM) technology. The validation of method was conducted under these assay parameters: including precision and accuracy, selectivity, linearity of dilution and stability. The assay sensitivity is 0.23438 pg/mL with MRD 1:4.
Method: In the first step, anti-IL-17A coated paramagnetic capture beads were incubated with diluted samples standards and QC samples in order to capture IL-17A. The beads were washed and incubated with a biotinylated detection antibody that binds to the captured IL-17A. Following a second wash, a conjugate of streptavidin-ß-galactosidase(SBG) was added to the sample. SBG will bind to the biotinylated detection antibody, resulting in the enzymatic labeling of the captured IL-17A. Following a third wash, the capture beads were resuspended in a resorufin ß-D-galactopyranoside (RGP) substrate solution and transferred to the Simoa Disc. Individual capture beads are sealed within the microwells of the array. Captured and labeled IL-17A will hydrolyzes the RGP substrate into a fluorescent product that provides the signal for measurement. A single labeled IL-17A molecule results in sufficient fluorescent signal in 30 seconds to be detected and counted by the Simoaoptical system. At low IL-17A concentration, the percentage of bead-containing wells in the array that have a positive signal is proportional to the amount of IL-17A present in the sample. At higher IL-17A concentration, when most of the bead-containing wells have one or more labeled IL-17A molecules, the total fluorescence signal is proportional to the amount of IL-17A present in the sample. The concentration of IL-17A in unknown samples are interpolated from a standard curve.
Conclusions: Method for quantitation of IL-17A in human serum samples has been validated using the Quanterix SimoaHD-1 Analyzer which has been validated following 21 CFR part 11. The LLOQ of the assay is 0.23438 pg/mL. The LQC evaluated in this validation was prepared at a concentration of 0.44012 pg/mL in human serum. The assay MRD is 1:4. The dynamic range of the method is 0.23438 -120 pg/mL. Results for accuracy, precision, mixed samples, stability and dilution linearity met the required acceptance criteria specified in the validation plan.
Find out more about the use of a Simoa Human IL-17A assay in this poster from Frontage Laboratories. To view the poster, please click here!
For more information on Frontage Laboratories, please click here!