What is chromatography and how does it work?

What is chromatography?
Chromatography is a technique carried out in laboratories to separate components into simple or complex mixtures. There are many different types of chromatography, ranging from paper chromatography and thin layer chromatography to gas chromatography.
Although there are many forms of chromatography, they all operate on the same principle. All the different types feature a stationary phase – usually a solid – and something to carry the complex mixtures through the stationary phase, called the mobile phase, which is usually a gas or a liquid.
The different types of chromatography can be categorized depending on the type of mobile phase used. If the mobile phase is liquid, the technique falls under the category of liquid chromatography. Likewise, if the mobile phase is gas, the technique falls under the category of gas chromatography.
You may also be interested in:
- End of the road? The future of chromatography
- The power of HPLC for endogeneous compound analysis
- Developing novel stationary phases and capillary columns – an interview with Joseph Pesek
- Does chromatography need mass spectrometry?
Usually, the stationary phase is a porous solid, for example, silica or alumina. The packing of the stationary phase varies depending on the type of chromatography being used. For example, in thin layer chromatography, it is common to have silica gel packed onto aluminum sheets as the stationary phase. In a column system, the stationary phase is often packed into a glass tube.
The mobile phase is used to carry the mixture that is being separated through the stationary phase. In the case of liquid chromatography, the mobile phase is a solvent or combination of solvents that the mixture is soluble in, for example, dichloromethane or ethyl acetate. In the case of gas chromatography, the mobile phase is an inert gas, for example, helium or nitrogen.
How does chromatography work?
All types of chromatography work upon the same basic principle. As the names suggest, the mobile phase is ‘mobile’ and flows through a ‘stationary’ stationary phase. As the moving mobile phase carries the mixture through the stationary phase, the individual components in the mixture are partitioned between the stationary and mobile phases. This process allows the separation of components in the mixture because different components in the mixture have different interactions with the stationary and mobile phases.
In practice, this means that components in the mixture that ‘hold on more tightly’ to the stationary phase, stay for longer on the stationary phase. This means that they are separated from other components in the mixture that do not ‘hold on as tightly’ to the stationary phase. This is the basis of chromatography as a separation technique.
Chromatography: step by step
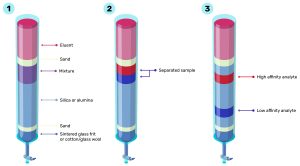
Figure 1. Simple liquid column chromatography shown in three stages; 1) assembly of the column, 2) continual addition of the mobile phase to begin separation and 3) separation of the components with high vs low affinity.
An example of a simple liquid column chromatography system is shown in Figure 1.
- Cotton wool or a glass sintered frit is placed at the bottom of a glass column and the column is packed with silica. There are different methods to pack the column, including dry packing or a silica slurry. The important factor is ensuring the silica is well packed, with no air bubbles or cracks as this will impede mobile phase flow. Once the silica is well packed, the mobile phase, often called the eluent, is added until it reaches the top of the silica. Following this, the mixture that is going to be separated is carefully added to the top of the saturated silica. A layer of sand is then added to the mixture to ensure it is not disturbed by the addition of more eluent.
- The eluent (the mobile phase), which can be a solvent or mixture of solvents, is then continuously added to the system until the mixture has been separated. It is important to make sure the eluent level never drops below the top of the silica to prevent air bubbles entering the stationary phase and impeding mobile phase flow.
- As the eluent is flushed down the column, components of the mixture that ‘hold on tightly’ to the stationary phase (in this case silica) will remain in the column for longer. Conversely, components that do not ‘hold on tightly’ to the stationary phase will elute from the column more quickly and can be collected as fractions. This process is continued until all the individual components have eluted from the column individually. The result is a separated mixture.
Chromatography can function as a separation technique because different components in a mixture have different attractions to the stationary and the mobile phase. The different attractions are due to the different properties of the components in the mixture. Silica, a common stationary phase, features Si-O-H bonds at the surface. Si-O bonds and hydroxyl groups mean that the surface of the silica gel (the stationary phase) is very polar, capable of forming hydrogen bonds and can also take part in van der Waals, dipole–dipole and dipole–induced dipole interactions.
As the complex mixture passes through the column, how fast the individual components of the mixture elute from the column will depend upon two features:
1) The extent to which each compound ‘holds on to’ the stationary phase. This depends upon interactions between the individual components and the silica gel.
2) How soluble the individual components are in the mobile phase (the eluent). This depends on the interactions between the components and the solvent system.
Although several individual components in the mixture may be very similar and even capable of hydrogen bonding to the silica in the stationary phase, it is highly unlikely that they will hydrogen bond to the silica and interact with the mobile phase in exactly the same way. In complex chromatographic separations, co-elution (where multiple components elute from the column simultaneously) can occur. Through extensive and careful variation of the mobile phase, trialing different solvent systems and varying the pH, even some of the most complex mixtures can be separated into their individual components using simple liquid column chromatography.
What is chromatography used for?
As we have touched upon, chromatography is a separation technique and therefore the applications are wide and varied. Many scientists will find themselves performing some kind of chromatography work at some point in many projects. Chromatography can also be used as a purification technique to isolate the desired reaction product from a mixture of impurities.
Chromatography is also used as an analytical technique. By separating a mixture into its individual components, you can isolate substances present in a sample and then subject them to further analyses. Some forms of chromatography can detect substances present at the attogram (10-18 gram) level, making it an accomplished trace analytical technique.
Chromatography is unparalleled as a separation technique and even finds application in the petroleum industry. In this industry, it is used to analyze the complex mixtures of hydrocarbons found in petroleum. In the bioanalytical field, chromatography is widely used for the separation and identification of chemical compounds and therapeutic drugs.
What is gas chromatography?
As with all forms of chromatography systems, gas chromatography is a technique that requires the use of a stationary phase and a mobile phase. In the case of gas chromatography, the mobile phase is an inert gas, often helium or nitrogen. The stationary phase is usually a microscopic layer of liquid or polymer on an inert solid or support. This is housed inside a coiled glass or metallic tube which is also called a column. At the end of this is a detection system that detects the individual components as they elute from the column.
How does gas chromatography work?
Gas chromatography is a common technique but can only be used when the mixture can be vaporized without decomposition due to the requirement of a gas mobile phase. Once the complex mixture has been vaporized, it is injected into the column along with the inert gas mobile phase.
Once the mixture is flowing through the column as a gas, the individual components in the mixture interact differently with the stationary phase. Like the column system described above, those components that do not interact as strongly with the stationary phase elute from the column more quickly and are detected by the detection system. Those that interact more strongly with the stationary phase take a longer time to elute from the column and therefore the mixture is separated.
The applications of liquid chromatography:
- Development and validation of a high performance liquid chromatography–MS/MS method for determination of SOMCL-15-290 in a first-in-human study
- Validation and application of hybridization liquid chromatography-tandem mass spectrometry methods for quantitative bioanalysis of antisense oligonucleotides
- Microspray and microflow liquid chromatography: the way forward for LC–MS bioanalysis