11th WRIB poster award winner: Neal Simmons

The Bioanalysis Zone and Bioanalysis team attended this year’s 11th Workshop on Recent Issues in Bioanalysis (WRIB; LA, USA), 3–7 April 2017.We were particularly proud to support and present the WRIB Poster Awards to Annelies Codden, John Lowe and Neal Simmons. Here, we feature the winning poster from Neal Simmons (AIT Bioscience) as well as an exclusive interview.
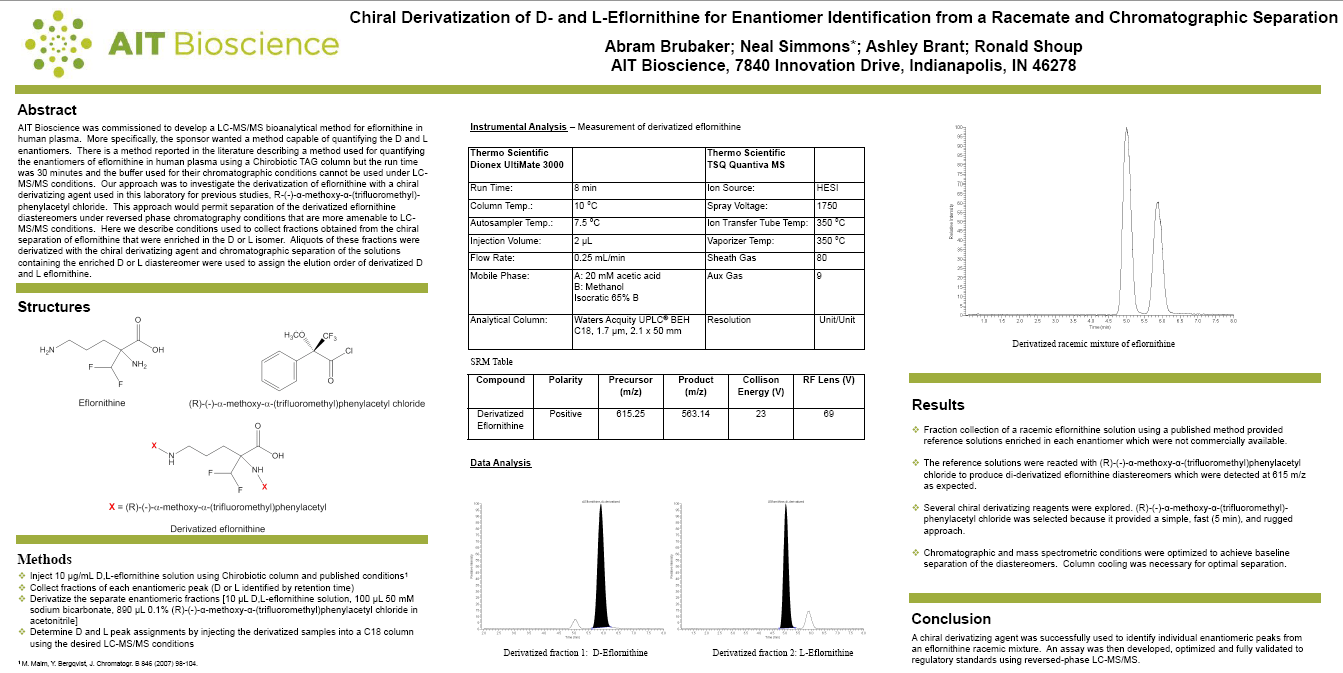
Click the image below, or click here to view the winning poster:
What the Judges had to say
“The winning poster from AIT Biosciences demonstrated a very interesting and innovative technique whereby a derivatizing reagent was used to convert an analyte enantiomeric pair to its diasteriomeric form, so that each isomer could be easily chromatographically separated then quantified by LC–MS/MS.” – Jeffrey X Duggan (Director, Bioanalytical Mass Spectrometry, Boehringer-Ingelheim Pharmaceuticals) & Olga Kavetska (Director, Group Lead, Clinical Assay, Pfizer, Inc.)
Interviw with Neal Simmons
Neal Simmons is a Senior Scientist II at AIT Bioscience (Indianapolis, IN USA). He received his Ph.D. in analytical chemistry from Iowa State University and has worked in bioanalytical labs at both large pharma and CROs for 20 years. He joined AITB in 2016 in the LC-MS/MS group and focuses on bioanalytical assay development and validation supporting regulated as well as research projects. Prior to AITB, he spent 9 years in a global pharma supporting clinical development programs through managing an in-house bioanalytical lab and outsourcing. His interests currently include anything unusual on the horizon plus tissues, derivatization, chiral assays, and delving into hybrid LBA-LC-MS.
1 Please can you explain about your role at AIT Bioscience?
Working at a CRO requires wearing many hats as you can imagine. My primary role is to support research and method development of LC-MS/MS assays mainly focusing on small molecule drugs and metabolites. In addition to the lab aspects, I also am a principal investigator/primary contact which means working closely with sponsors on planning, conducting, and final reporting of their projects.
2What were your first thoughts on winning the 11th WRIB Poster Award?
When the poster awards were announced, I was completely surprised. There were many great posters and research topics presented so it was totally unexpected. I was thrilled to accept as the presenting author, but the work was very much a team effort. This was a project that could have gone in many directions and become extremely complicated, but instead had a very simple and elegant solution. We all appreciated the recognition from the panel as well as the comments and discussions with those who stopped by during the poster session earlier.
3 How did you carry out the work highlighted in this poster?
During the planning stage, we knew that the entire project hinged on one key question. How could a rugged method be developed to measure the D- and L- enantiomers of eflornithine when enantiomeric reference standards are not currently available? To address the ruggedness issue, Abe decided to look at chiral derivatization which would form a pair of diastereomers that could then be measured by reversed-phase LC-MS/MS. A similar approach was used in our lab a few years ago for chiral amphetamine analysis and it worked well. The final hurdle, and heart of the poster, was assigning the proper identity, D- or L-, to the resulting chromatographic peaks without standards. This was solved by preparing our own standards using a published chiral eflornithine assay for fraction collection of the enantiomers from a racemic standard.
4 What were the key findings from your research?
Derivatization, chiral in this case, can sometimes provide a simple solution to a complex problem. The poster results and conclusions demonstrate that this approach worked well for measuring chiral eflornithine using standard reversed-phase LC-MS/MS conditions. After surveying derivatizing agents, conditions, and pre-/post-derivatization cleanup, we found that the most effective processing was to perform a protein precipitation of plasma and then directly derivatize the supernatant. After a 5-minute reaction, samples were evaporated to dryness then dissolved in an injection solvent. For confidentiality reasons, we could not present the validation details; however, the method demonstrated excellent statistics and chromatography. This was particularly important/surprising when you consider the simplicity of the final procedure. Finally, the assay was set up multiple times over the last 6 months with no difficulties, demonstrating overall ruggedness of this methodology.
5What are you looking forward to working on over the next year?
We have quite a few unusual projects either queued up or under discussion which involve topics covered at WRIB this year. These include the challenges of assay development for ocular fluids and tissues as well as hybrid LBA-LC-MS. If there are suitable projects, I am also looking forward to expanding my derivatization experience.
You can view posters and interviews from the other award winners here.