LBA for biomarker validation: an interview with Joel Mathews
In this exclusive interview with Bioanalysis Zone, Joel Mathews (Ionis Pharmaceuticals Inc., CA, USA) discusses his role in the development of biomarker, PK and immunogenicity assays in the development of protein therapeutics. He explains his research background and interest in the field as well as exploring the use of LBA for biomarker validation. Key questions that Joel answers include:
- What are the basic principles of LBA?
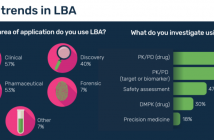
- How do you use LBA for biomarker validation?
- What are the main advantages of LBA compared to other techniques?
- What lessons have you learnt when using LBA for biomarker validation?
- Where do you hope this field will be in 5–10 years’ time?
If you enjoyed this, you may also be interested in..
LBA critical reagent characterization: an interview with Jon Haulenbeek