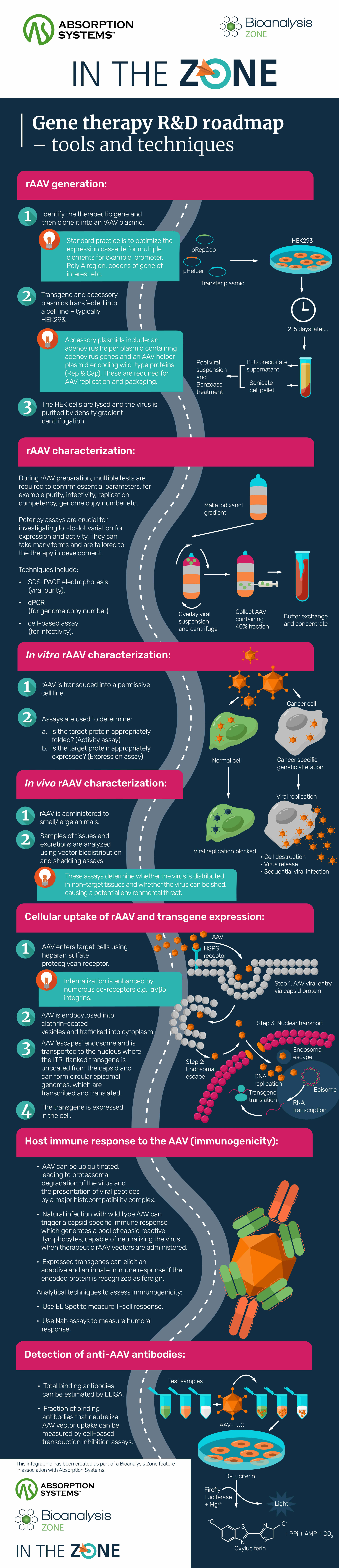
This In the Zone infographic explores the tools and techniques used during the AAV gene therapy development pathway, detailing the neccessary steps during the process; from rAAV generation and in vitro and in vivo testing through to assessing the host immune response.