DBS is sound science but also sound economics
DBS is a very promising approach for collecting exposure samples for a clinical study. The main obstacle today seems to be that we do not know if relevant authorities will understand and accept the approach of DBS, even in a fully validated application. Whether DBS can be the future standard approach in sample handling, depends of course, on the physical and chemical properties of the DBS.
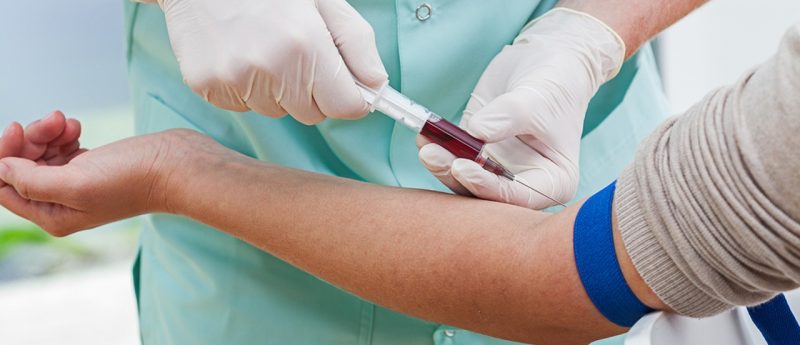
Ever since I joined the bioanalytical community in the mid-1970s, there has been an issue of how to handle plasma samples i.e. how to secure the dispatch of samples, how to store samples, how to thaw samples, how to evaluate hemolytic samples, how to dispose of sample surplus etc.
We do not yet know the end of the DBS story, but what has been reported so far looks very promising. It seems that this ’novel‘ approach matches deep frozen plasma, in most respects. Today, we have a limited experience of DBS compared with frozen plasma, but from what I have seen in the laboratory so far I am full of expectation when it comes to future DBS applications.
When we consider sample collection and sample dispatch to a central bioanalytical laboratory, DBS minimizes the costs, and the simplicity gives increased quality. Let us for example assume a clinical Phase III study comprising 1200 subjects. It would be reasonable to collect five samples per subject on average, from 200 clinical sites.
Additionally, we cannot put the bioanalysis on hold until the study is complete i.e. we must at least dispatch samples at three occasions from each clinical site. This means 6 000 samples divided in 600 dispatches. If we assume that every dispatch of deep frozen plasma on dry ice with a temperature log costs 1.000 Euro, this totals 600.000 Euro. If we assume that one target analysis at a CRO costs 70 Euro, the bioanalytical cost totals 420.000 Euro. This means that in an ordinary clinical Phase III study, the cost for the bioanalysis is lesser than the cost for the dispatches of the samples. If we apply the DBS approach, we could simply put the samples in the mailbox at no cost at all.
When we compare the routines for collecting plasma samples versus DBS, we notice that the DBS routine is far simpler. The routine for collecting plasma starts with sampling whole blood in a heparin test tube. The plasma is then separated by means of centrifugation and put on dry ice awaiting transferee to a deep freezer. In this routine there are several opportunities to make mistakes , for example, the sample identity labeling from whole blood to plasma is corrupted, there is hemolysis in the whole blood, the drug is degraded during centrifugation, the test tube is forgotten in the centrifuge for a period of time or the labeling is not resistant to condensed humidity. It is much simpler to put a drop of blood on a paper labeled with subject, date, time etc. This will almost by definition increase quality.
I have fully investigated DBS in one application (to be published) and found that DBS in this application fully matches frozen plasma when it comes to:
- Method development and validation
- Method yield and limit of detection
- Sample throughput
- Storage stability of spiked samples
- Reanalysis of incurred samples
- Stability of incurred samples.
And I also recognize that DBS overthrows frozen plasma when it comes to:
- The collecting and the labeling of samples
- The dispatch and reception of samples
- Sample preparation to an analytical extract
- Matrix simplicity (LC–MS/MS analysis).
Let us be optimistic and assume that if the DBS approach is fully accepted and understood by QA departments and regulatory authorities, and that the scientific rational holds, this will simplify life in the bioanalytical community and increase data quality.





