Chapter 2: Advances in LC separations for proteomics
Doi: 10.4155/FSEB2013.14.373
Authors
Yet-Ran Chen
Associate Research Fellow/Associate Professor
Agricultural Biotechnology Research Center
Academia Sinica
128 Academia Rd. Sec. 2, Nankang, Taipei, Taiwan (R.O.C.)
Tel: 886-2-2787-2050
e-mail: [email protected]
About the Authors
Yet-Ran Chen is an Associate Research Fellow of Agricultural Biotechnology Research Center at Academia Sinica and an Associate Professor of the Institute of Biotechnology at National Taiwan University in Taiwan. His research focuses on the development of new platform technologies based on mass spectrometry for the investigation of cell–cell communication dynamics.
Advances in LC separations for proteomics
The complexity and broad dynamic range of the proteome presents great analytical challenges in global proteomics investigations. Because of its high sensitivity, high resolving power and the robustness of the instrumentation for peptide analysis, liquid chromatography-mass spectrometry (LC-MS) has developed into the main proteomics research platform. Although the sensitivity of MS for peptide identification has been improved to the sub-attomole level, LC-MS is still not capable of detecting all cellular proteins in a typical mammalian cell lysate. This is owing to the high complexity and dynamic range of the global proteome making the detection of low abundant peptides highly challenging. In addition to the performance of MS, chromatographic efficiency is considered to be a critical factor for the detection of low abundant proteins. In this chapter, the basic concepts and major advances in current LC separation technologies for proteomics are introduced and discussed.
Nanoflow LC-MS (nanoLC-MS) for proteomics
One of the major breakthroughs in proteomics is the development of online nanoflow LC-MS (nanoLC-MS) systems for peptide identification, and this is now the major platform for proteomics research. Recent developments in nanoLC have dramatically improved overall LC-MS performance for the analysis of complex cellular proteomes.
Why nanoLC-MS?
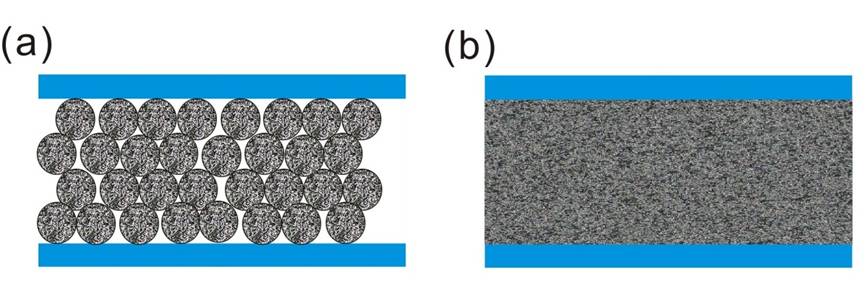
Electrospray ionization (ESI) is the most common ionization and interfacing method for LC-MS analysis of peptides and proteins. The ESI source can directly couple to the outlet of the LC column and efficiently ionize the eluted proteins/peptides from the liquid phase. The reduction of the optimal ESI flow rate can produce smaller initial ESI droplets and electrospray plume size, thus producing better de-solvation, ionization and MS sampling efficiency. It has been demonstrated that nanoflow ESI (nESI) can increase sensitivity more than 50-fold over standard ESI. In the LC analysis, the reduction of the column diameter means not only can the column be directly coupled to a more sensitive nESI interface, the concentration effect of the analytes on the column is also significantly increased. The concentration factor for a narrow-bore HPLC column is ~100-fold, but the capillary column can reach ~1000-fold in concentration enrichment. Since ESI is a concentration-dependent ionization technology, the online coupling of nanoLC to nESI-MS can result in 100–1000-fold higher sensitivity than the coupling of LC with ESI-MS. Currently, both of the major LC column categories, packed and monolithic (continuous bed) column design, have been applied in nanoLC separations and are widely used in proteomics studies (Figure 1).
Figure 1: (a) Packed and (b) monolithic column designs.
Packed nanoLC column
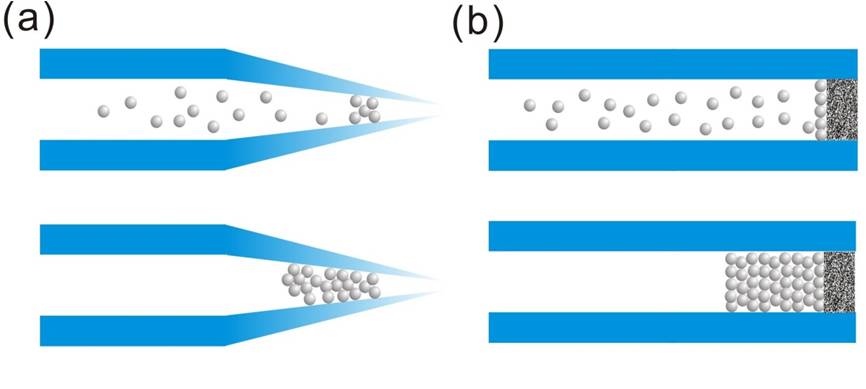
Because of the ease and high reproducibility of column preparation, packed nanoLC columns are still the major separation method in proteomics. The key to fabricating a packed nanoLC column is the retention of packing particles. Most self-prepared nanoLC columns are made by packing particles into a tapered tip or a commercial capillary with an integrated outlet frit (Figure 2).
Figure 2: The particle packing in (a) tip columns and (b) frit columns.
Tip-packed column
In the tapered tip capillary, one end of the column is heat-drawn to create a tapered tip with an outlet diameter smaller than the capillary internal diameter (ID). The reduction of the capillary ID in the column makes particles aggregate at the tapered end during the particle packing process without using a physical barrier or filter. This particle aggregation acts as a “keystone” blocking the other particles, thus allowing the packed section to grow. The aggregation phenomenon cause by the particles flowing through a tapered channel is called the “keystone effect” [1], as illustrated in Figure 2a. This effect was shown by tapering a conventional 75-µm ID capillary to 10-µm ID, which was sufficient to make 3-µm particles aggregate at the tapered end of the capillary [2]. The tapered tip of the column also functions as an emitter of nESI. The fabrication of the tapered tip capillary is simple and minimizes the post-column dead volume, which reduces band broadening due to longitudinal diffusion. The major drawback of this design is that when the tip is cracked or electron etched the whole column has to be discarded and replaced with a new one to keep the optimal sensitivity and signal stability of nESI. Because the outer diameter is reduced at the tapered end, the column outlet cannot connect to other fittings, thus the tapered tip capillary cannot be used in the fabrication of trap columns or be the first column in multi-dimensional liquid chromatography (MDLC) systems.
Frit-packed column
A capillary with an internal frit can be used in the preparation of trap columns; this design is also commonly used for the preparation of analytical columns. For the application of analytical frit columns to nanoLC, a short tapered tip capillary is coupled to the outlet of the analytical column as the nESI emitter using a zero dead volume union. In this design, the nESI emitter or analytical column can be replaced independently when the ionization or separation efficiency drops. The fabrication of in-capillary frits is challenging because the frit should not only have enough strength to support the packing material, but also have high permeability for liquid flow. Several methods are used for the fabrication of in-capillary frits. Most of the commercial frit capillaries are fabricated by sintering glass beads inside the capillary [3]. The sintering process requires high temperature to melt the silica beads and results in the loss of the polyimide layer at the capillary end. The removal of the polyimide layer can cause the column end to be susceptible to mechanical stress. Sol-gel frit capillaries can be fabricated without sintering, and thus preserve the polyimide coating for better robustness. However, the liquid permeability of sol-gel frits is not reproducible and they may be crashed or produce significantly high back pressures during liquid flow. To improve frit fabrication reproducibility and robustness of the frit column, the development of a tunnel frit was reported recently [4]. The tunnel frit was fabricated by inserting a metal wire during the sol-gel polymerization reaction and the wire was removed after the polymerization reaction, thus generating a sol-gel frit with a tunnel. Because there is a channel in the frit, the sol-gel porous structure is no longer so important for liquid permeability. The way in which the tunnel frit retains the particle is the same as the tip column does, which is by engaging the keystone effect. Because the optimal sol-gel porous structure is not required for this frit, the fabrication reproducibility of the tunnel frit is no longer controlled by the sol-gel polymerization reaction. This design has been demonstrated to tolerate liquid pressures higher than 11,000 psi and two ends of the column can be connected to fittings for ultra-high performance liquid chromatography (UHPLC).
Packing materials for proteomics applications
Reversed-phase liquid chromatography (RPLC) is the most common separation method for complex peptide mixtures. The resolution of peptide separation is affected by the particle geometry, pore size, alkyl ligand and silanol endcapping. Silanol endcapping is important for the separation of peptides because the N-terminal and basic residues of the peptide are positively charged in the acidic mobile phase. The positively charged peptides can produce ionic interactions with the uncapped surface of the silica gel. This secondary ionic interaction in the RPLC column reduces the separation resolution. TFA is a common buffer additive in RPLC to avoid the interaction of charged residues with silanol groups, so improving the separation resolution of peptides in RPLC. However, TFA reduces the MS detection sensitivity for the peptides. In LC-MS analysis, TFA is replaced by formic acid (FA) as the additive in the mobile phase. Because FA is a weak ion pairing reagent, the uncapped surface of the base particle becomes more critical in the separation efficiency when FA is used in the mobile phase. The recent development of ethylene-bridged hybrid (BEH) packing materials has provided additional improved options [5]. Silica-based hybrid organic/inorganic packing materials are synthesized using bis(triehtoxysilyl)ethane and tetraethoxysilane. The ethylene bridges reduce the surface concentration of silanol groups to minimize the secondary interactions, especially when FA is used as the mobile phase additive. It has been demonstrated that there is no significant difference in peptide separation efficiency when either TFA or FA are used in RPLC with BEH-based columns. The development of BEH-based columns has also improved alkyl ligand stability at higher pH. The change of mobile phase pH can dramatically alter the separation specificity and this provides additional flexibility for the optimization of peptide separations.
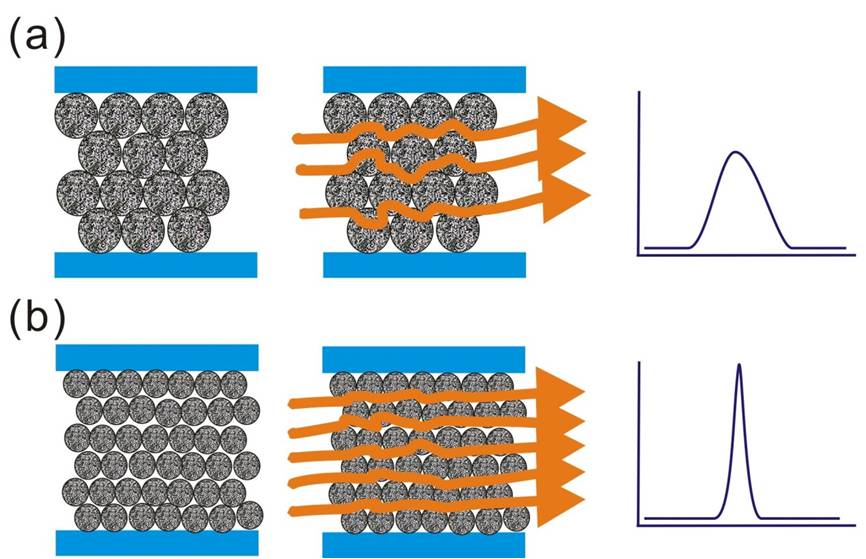
The geometry of the packing materials is also an option for the optimization of separation efficiency. The van Deemeter equation reveals that the mass-transfer coefficient in the liquid phase is a function of the square of the diameter of the packing particles. The higher mass-transfer coefficient and lower eddy diffusion effect by reduction of the particle size should dramatically reduce the plate height and thus improve the column efficiency (Figure 3). In addition, the reduction of the particle size also increases the optimum linear velocity at which the minimum plant height is achieved, so a higher flow rate can be used to reduce separation time without compromising in the separation efficiency. Based on this concept, the new-generation separation technology UHPLC was developed by packing sub-2-µm particles into the column for obtaining better separation efficiency (140,000 < N < 190,000) with a shorter separation time [6]. The sub-2-µm packing particles can produce very high backpressure, which is in the range of 8000–15000 psi. Sensitivity is also improved in UHPLC because each of the analytes is subject to less peak broadening during the separation and this produces sharp chromatography peaks of higher peak height. The improved performance of using smaller packing particles was demonstrated by analysis of a digest a soluble fraction from yeast where ∼30% more protein identifications were achieved using a 60-cm long triphasic capillary column than with the traditional approach [7]. More recently, UHPLC has been used to profile core histone modifications from primary human fibroblasts. The whole analysis was performed on intact unfractionated histones within 19 min, which is ∼threefold faster than conventional procedures [8]. The UHPLC operating pressure is higher than the pressure limits of standard HPLC systems and fittings, thus most of the components in the LC systems for UHPLC were redesigned for the purpose.
Figure 3: Schematic representation of the flow path in a packed column with (a) large or (b) small packing particles.
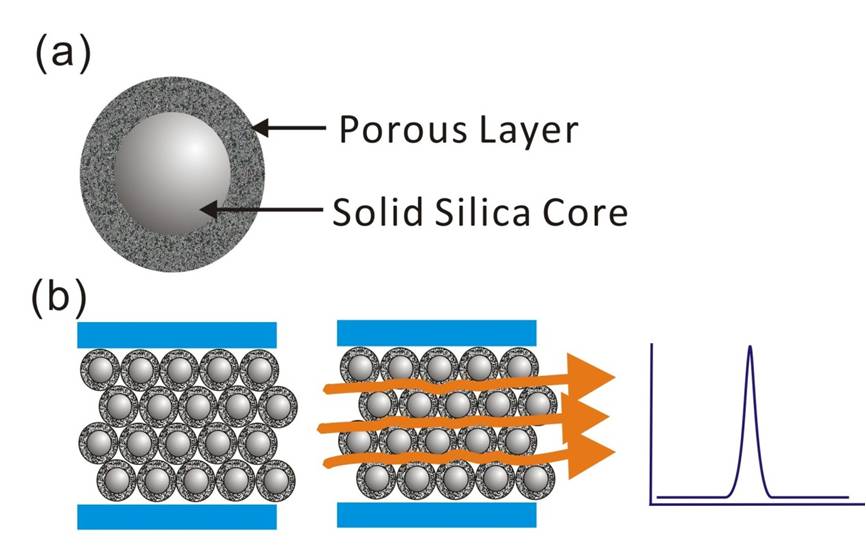
In addition to the development of UHPLC, another breakthrough was the development of core–shell particles for LC separation. In core–shell particles, a uniform porous layer is grown around a spherical solid silica core (Figure 4a). This design can increase the rate of mass transfer by decreasing the effect of diffusion inside of the particle (Figure 4b). The core–shell can produce nearly monodispersed particle size for diminished Eddy effusion effects in the LC column. The first proposed version of the core–shell was almost 50 years ago but this design was limited by low loading capacity, poor uniformity and mechanical stability of the porous area. New-generation core–shells were developed by generating durable and homogeneous porous shells on the solid silica core using sol-gel processing techniques [9]. By taking advantage of this technology, the core–shell packing particles now provide high loading capacity, durability and narrow particle size distribution. A chromatographic column packed with 2.6-µm core–shell particles produces UHPLC-like separation efficiency using porous sub-2-µm particles [10]. Columns packed with 2.6-µm core–shell particles offer significant advantages over columns packed with fully porous sub-2-μm particles, because the column pressure is in the range of typical HPLC systems.
Figure 4: The (a) packing particle and (b) the flow path of a core–shell packed Column.
Monolithic nanoLC Columns
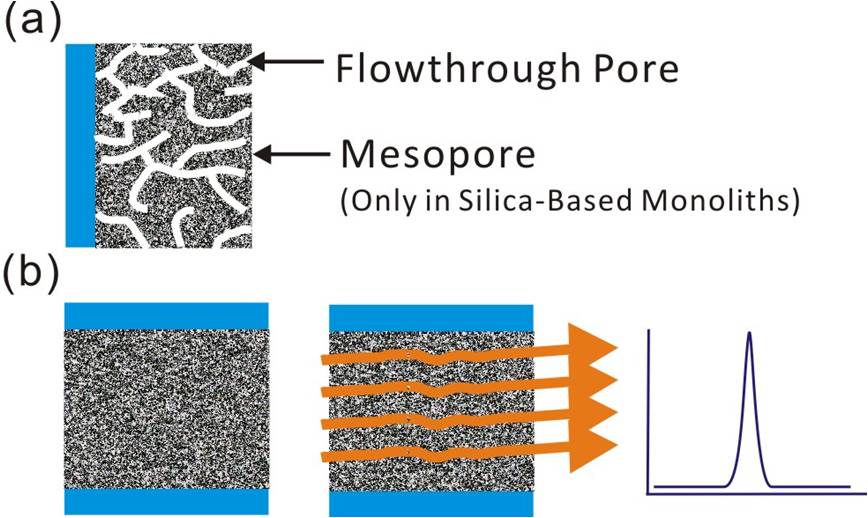
Monolithic columns have been developed to archive rapid and efficient separation performance using conventional HPLC instrumentation. The monolithic column is a type of column with continuous porous beds, no frit required. There are two types of monolithic column, one is silica-based monoliths and the other is polymer-based monoliths. The polymer based monolithic columns are composed of large flow-through pores, which are suitable for the separation of macromolecules. The silica-based monoliths have bimodal structures composed of small mesopores and larger flow-through macropores, as shown in Figure 5. The mesopores produce a high surface area and this surface is chemically modified to define the separation mechanism. The macropores in the column result in the high permeability of the structure, to which high liquid flow rates can be applied without dramatically elevating column backpressure. Because the porous specific surface area of silica-based monoliths is ~two orders higher than that of the polymer-based monoliths, the silica-based monoliths are more commonly applied to reversed-phase nanoLC columns for peptide separations. Unlike packed columns, the silica-based monolithic columns do not have interstitial void spaces and thus have minimized longitudinal and eddy diffusion effects, so can produce high separation efficiency comparable to UHPLC columns packed with sub-2-µm particles. Moreover, the silica-based monolithic columns have high pore connectivity allowing peptide molecules to diffuse into one pore and exit through any of 6–10 different channels [11]. Most of the porous beds in a silica-based monolithic column can interact with the peptides. The monolithic column is especially suitable for the analysis of proteins or peptides, because the nm-scale porous structure can allow macromolecules such as peptides or proteins to access the internal bed. For the analysis of peptides, the binding capacity of the silica-based monolithic columns is almost 10-fold higher than that of full porous bed packed columns [11]. Because of the low backpressure, it was demonstrated recently that a nanoLC-MS platform with 4-m long and 100-µm i.d. C18 modified silica-based monolithic column was able to support the identification of almost 6,000 proteins from 4 µg of human HeLa cell lysate reproducibly (in quadruplicate measurements) [12]. In general, the C18 modified silica based monoliths are fabricated by first establishing a silica skeleton by mixing methyltrimethoxysilane with tetramethoxysilane followed by C18 modification of the silica surface using octadecyldimethyl-N,N-diethyl aminosilane [13]. The free silanol groups can be capped by n-(trimethylsilyl)imidazole to minimize the nonspecific adsorption of peptides [12]. The major limitation of the silica-based monolithic columns is their tolerance to high pH. Polymer monolithic columns can be operated in low and high pH ranges; however, they are limited by low specific area because they lack mesopores. There have been several hybrid monoliths designs proposed recently, which can result in high specific area and pH-stable columns. However, their biocompatibility and performance when used for the analysis of complex proteomes still need to be demonstrated [14].
Figure 5: The (a) pore Structure and (b) flow path of a monolithic column.
Multidimensional LC-MS (MDLC-MS) for complex proteome analysis
To date, there is no single separation method that provides sufficient peak capacity to resolve all the peptides and proteins in complex biological samples. The multidimensional separation approach is an efficient strategy to increase the peak capacity for complex mixtures. The enhancement of peak capacity by coupling multiple separation methods is determined by the degree of separation orthogonality. In MDLC-MS, the last separation method is commonly RPLC because the mobile phase used in RPLC is compatible with ESI-MS. In addition, the low pH and organic solvents used in RPLC can enhance ESI sensitivity and stability for detection and quantification of the peptides eluted directly from the column. Therefore, in MDLC-MS analysis, the first separation dimension is considered by the separation orthogonality to the RPLC in acidic separation conditions.
Ion exchange-reversed phase (IEX-RP) chromatography
The most widespread separation method used in the first dimension for MDLC is strong cation exchange (SCX) because it provides good orthogonality to RPLC. In SCX-RP analysis, the SCX generally utilizes salt gradients to separate the peptides and the addition of the organic solvent minimizes hydrophobic interactions during the separation. However, the organic solvent can prohibit the RPLC from trapping the peptides and the high salt in the SCX eluent may affect the MS detection if SCX is directly coupled to the RPLC-MS. So SCX is generally coupled offline with RPLC-MS to allow the organic solvent and the salt in the SCX eluent to be removed before injection on the RPLC. The SCX is considered not completely orthogonal to the RP, because the SCX separates peptides according to their charge state in solution, but the number of charges for tryptic peptides is normally in a very narrow range (2+ to 4+) [15]. With the combination of strong anion exchange (SAX), SCX and RP separations, the online “yin-yang” MDLC-MS system was developed for comprehensive proteome and phosphoproteome profiling without any prefractionation or chemical derivation [16]. In “ying-yang” MDLC, the basic peptides are separated by SCX, whereas the acidic peptides are collected as flow-through and further separated by SAX. Phosphopeptides are mainly retained in SAX because they are more acidic. All the peptides separated from SCX and SAX columns are then subjected to RPLC separation and MS identification. Phosphopeptides and non-phosphopeptides can be quantified concurrently using “ying-yang” MDLC-MS, which has been applied successfully to systematically reveal the transcriptional regulation events during the initial period of adipocyte differentiation [17].
Hydrophilic interaction chromatography-reversed phase (HILIC-RP) chromatography
Hydrophilic interaction chromatography (HILIC) has been demonstrated to be a better orthogonal separation method for RPLC-MS [18]. HILIC has recently become the new method of choice for the analysis of hydrophilic and ionic solutes. The mobile phase of HILIC is considered a modification of normal-phase liquid chromatography because both technologies utilize polar stationary phases. The mobile phase used in HILIC is different from the normal phase separation because it is a water-miscible organic solvent. Unlike RPLC using high aqueous solvent to facilitate peptide trapping, HILIC requires a high percentage of organic solvent for trapping. The high percentage of organic solvent in the HILIC column generates a water-enriched layer partially immobilized on the surface of the stationary phase. Retention in HILIC is described as a mixed-mode mechanism, in which partitioning of the analyte between the organic-rich mobile phase and the water-enriched layer is regarded as the main retention mechanism [19]. The mobile phase system used in the HILIC is the same as that used in the RPLC; this makes the whole LC configuration more compatible to the MS analysis. The peptides eluted from the HILIC column cannot be readily analyzed by RPLC; this is owing to the HILIC eluents containing a high concentration of organic solvent. It is necessary to evaporate or dilute the organic solvent in the HILIC eluent to less than 10% before injection onto the RP column. It is also possible to online couple HILIC and RP; the major requirement is to mix the HILIC eluent with water, pumped by a second pump, and inject the mixed eluent to the RPLC [20].
Electrostatic repulsion-hydrophilic interaction chromatography–reversed phase (ERLIC-RP) chromatography
Electrostatic repulsion-hydrophilic interaction chromatography is a variation of HILIC, the major difference being the use of ion-exchange column with the same charge as most of the analytes [21]. This method was applied to enrich phosphopeptides, fractionate N-linked glycopeptides, and simultaneously enrich glyco- and phospho-proteomes from a complex sample. As in HILIC, the high concentration of organic mobile phase in ERLIC can retain the analytes in the aqueous layer around the stationary phase through hydrophilic interaction even when the analyte has the same charge as the stationary phase. The superimposed electrostatic repulsion and hydrophilic interaction antagonize each other’s retention, thus ERLIC allows the isocratic separation of complex peptide mixtures. Because electrostatic and hydrophilic interactions are used in ERLIC separations, both of which show good orthogonality to RP, ERLIC-RP has shown better protein identification performance than SCX-RP [22].
Reversed phase-reversed phase (RP-RP) chromatography
In considering the ease of use and robustness of an MDLC system, RP-RP MDLC was proposed by using two RP columns separated by two mobile phase systems with different pHs. This has the potential to be effective because the RP separation selectivity is strongly affected by the mobile phase pH owing to alteration of the charge distribution of peptide acid and base residues. It has been demonstrated that an RP-RP system using buffer systems at pH 2.6 and pH 10 provides reasonable orthogonality in MDLC separations [23]. At pH 2.6, the side chains of the acid residues Asp and Glu are nearly neutral, thus moderately hydrophobic, but at pH 10 they are almost completely deprotonated and are negatively charged. In contrast, the basic residues Arg, Lys and His are positively charged at pH 2.6 but nearly neutral at pH 10. The high-pH column binds basic peptides more strongly and acid peptides are retained to a greater extent in a low-pH column. The conventional silica-based RP packing material shows low stability at high pH, which is not suitable for long-term operation. This RP-RP approach has been facilitated by the development of columns that remain stable at high pH. BEH packing material is now commonly used in RP-RP because BEH is stable between pH 2-11, and tolerant to low and high pH switching and separation (~5000 times) without reducing separation performance [24].
Financial and competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Executive Summary
- Packed nanoLC columns are made by packing particles into a capillary that are retained by the “keystone” effect or a physical filter.
- New generations of packed nanoLC use silica-based hybrid organic/inorganic packing materials to minimize secondary ionic interactions and the development of sub-2-µm packing particles or core–shell particles has enhanced mass transfer rates of the stationary phase.
- Monolithic columns are especially suitable for the analysis of proteins or peptides because the nm-scale porous structures can allow macromolecules, such as peptide or proteins, to access the internal column bed.
- In contrast to strong cation exchange (SCX), several new separation methods show better orthogonality to RPLC, are easy to operate and are compatible with nanoLC-MS.
References
- Ceriotti L, De R, Verpoorte E. An integrated fritless column for on-chip capillary electrochromatography with conventional stationary phases. Anal. Chem. 74(3), 639–647 (2002).
- Lord GA, Gordon DB, Myers P, King BW. Tapers and restrictors for capillary electrochromatography and capillary electrochromatography-mass spectrometry. J. Chromatogr. A 768(1), 9–16 (1997).
- Carney RA, Robson MM, Bartle KD, Myers P. Investigation into the formation of bubbles in capillary electrochromatography. J. High Resolut. Chrom. 22(1), 29–32 (1999).
- Chen CJ, Chen WY, Tseng MC, Chen YR. Tunnel frit: a nonmetallic in-capillary frit for nanoflow ultra high-performance liquid chromatography-mass spectrometryapplications. Anal. Chem. 84(1), 297–303 (2012).
- Wyndham KD, O’gara JE, Walter TH et al. Characterization and evaluation of C18 HPLC stationary phases based on ethyl-bridged hybrid organic/inorganic particles. Anal. Chem. 75(24), 6781–6788 (2003).
- Russo R, Guillarme D, D TTN, Bicchi C, Rudaz S, Veuthey JL. Pharmaceutical applications on columns packed with sub-2 microm particles. J. Chromatogr. Sci. 46(3), 199–208 (2008).
- Motoyama A, Venable JD, Ruse CI, Yates JR, 3rd. Automated ultra-high-pressure multidimensional protein identification technology (UHP-MudPIT) for improved peptide identification of proteomic samples. Anal. Chem. 78(14), 5109–5118 (2006).
- Contrepois K, Ezan E, Mann C, Fenaille F. Ultra-high performance liquid chromatography-mass spectrometry for the fast profiling of histone post-translational modifications. J. Proteome Res. 9(10), 5501–5509 (2010).
- Yu M, Lin J, Fang J. Silica spheres coated with YVO4: Eu3+ layers via sol-gel process: A simple method to obtain spherical core-shell phosphors. Chem. Mater. 17(7), 1783–1791 (2005).
- Olah E, Fekete S, Fekete J, Ganzler K. Comparative study of new shell-type, sub-2 micron fully porous and monolith stationary phases, focusing on mass-transfer resistance. J. Chromatogr. A 1217(23), 3642–3653 (2010).
- Bayram-Hahn Z, Grimes BA, Lind AM et al. Pore structural characteristics, size exclusion properties and column performance of two mesoporous amorphous silicas and their pseudomorphically transformed MCM-41 type derivatives. J. Sep. Sci. 30(18), 3089–3103 (2007).
- Iwasaki M, Sugiyama N, Tanaka N, Ishihama Y. Human proteome analysis by using reversed phase monolithic silica capillary columns with enhanced sensitivity. J. Chromatogr. A 1228(0), 292–297 (2012).
- Miyamoto K, Hara T, Kobayashi H et al. High-efficiency liquid chromatographic separation utilizing long monolithic silica capillary columns. Anal. Chem. 80(22), 8741–8750 (2008).
- Liang Y, Zhang L, Zhang Y. Recent advances in monolithic columns for protein and peptide separation by capillary liquid chromatography. Anal. Bioanal. Chem. 405(7), 2095–2106 (2013).
- Hu L, Ye M, Jiang X, Feng S, Zou H. Advances in hyphenated analytical techniques for shotgun proteome and peptidome analysis–a review. Anal. Chim. Acta 598(2), 193–204 (2007).
- Dai J, Jin WH, Sheng QH, Shieh CH, Wu JR, Zeng R. Protein phosphorylation and expression profiling by Yin-yang multidimensional liquid chromatography (Yin-yang MDLC) mass spectrometry. J. Proteome Res. 6(1), 250–262 (2007).
- Wu YB, Dai J, Yang XL et al. Concurrent quantification of proteome and phosphoproteome to reveal system-wide association of protein phosphorylation and gene expression. Mol. Cell. Proteomics 8(12), 2809–2826 (2009).
- Gilar M, Olivova P, Daly AE, Gebler JC. Orthogonality of separation in two-dimensional liquid chromatography. Anal. Chem. 77(19), 6426–6434 (2005).
- Greco G, Grosse S, Letzel T. Study of the retention behavior in zwitterionic hydrophilic interaction chromatography of isomeric hydroxy- and aminobenzoic acids. J. Chromatogr. A 1235, 60–67 (2012).
- Louw S, Pereira AS, Lynen F, Hanna-Brown M, Sandra P. Serial coupling of reversed-phase and hydrophilic interaction liquid chromatography to broaden the elution window for the analysis of pharmaceutical compounds. J. Chromatogr. A 1208(1–2), 90–94 (2008).
- Alpert AJ. Electrostatic repulsion hydrophilic interaction chromatography for isocratic separation of charged solutes and selective isolation of phosphopeptides. Anal. Chem. 80(1), 62–76 (2008).
- Hao P, Guo T, Li X et al. Novel application of electrostatic repulsion-hydrophilic interaction chromatography (ERLIC) in shotgun proteomics: comprehensive profiling of rat kidney proteome. J. Proteome Res. 9(7), 3520–3526 (2010).
- Dowell JA, Heyden WV, Li L. Rat neuropeptidomics by LC-MS/MS and MALDI-FTMS: Enhanced dissection and extraction techniques coupled with 2D RP-RP HPLC. J. Proteome Res. 5(12), 3368–3375 (2006).
- Acevska J, Dimitrovska A, Stefkov G, Brezovska K, Karapandzova M, Kulevanova S. Development and validation of a reversed-phase HPLC method for determination of alkaloids from Papaver somniferum L. (Papaveraceae). J. AOAC Int. 95(2), 399–405 (2012).