Robert MacNeill: charge state whack-a-mole
In this column from Robert MacNeill (Absorption Systems, PA, USA), he discusses how moles are a chemist’s friend and why all charge states should be monitored and used for quantification via summing.
Robert MacNeill received his Bachelor’s degree with Honors in Chemistry from Heriot Watt University then his MSc in Analytical Chemistry from the University of Huddersfield, both in the United Kingdom. Robert is also a Chartered Chemist and a Fellow of the Royal Society of Chemistry. With 20 years of experience in all aspects of quantitative bioanalytical LC–MS/MS method development, 11 of these years heading method development activities within HLS/Covance, and a regular author and peer reviewer for the journal Bioanalysis, Robert is a recognized expert and innovator in the field.
In his current role Robert coordinates all LC–MS/MS method development and associated training, takes the lead in keeping abreast of innovation and technological development in the industry, leads in-house research projects and performs technical writing for the purpose of producing publications.
Heads-up! Moles are naturally a chemist’s friend, wonderful close associates in quantitative bioanalysis. Although my father used to complain of excess ‘molarity’ digging around in the garden back home a few decades ago. Why would we want to play whack-a-mole? The analogy I attempt to make here is to do with the mass spectrometry of biologics and their innate multi-charging propensity. So, in assays for peptidic or protein analytes, or for nucleic acids, the appearance of several peak clusters corresponding to different charge states, and how the relative intensity can vary according to certain parameters, is likened to moles popping up from their holes and requiring to be analytically accounted for, or ‘whacked.’ In other words, how do we play charge state whack-a-mole with the best anticipation in consideration of the associated LC operation and the mass spectral interface in a quantitative bioanalytical LC–MS context?
In my opinion, the analogy to whack-a-mole helps to garner an understanding as to why all charge states should be monitored and used for quantification via summing. I appreciate the reasonable train of thought behind sticking to one transition in tandem mass spectrometry – the most intense – as the signal-to-noise will end up much the same, maybe less if summing was to be employed. My answer to that lies in the variability in abundance that inevitably occurs due to the influence of several experimental and functional bioanalytical LC–MS parameters. If abundance funnels from one charge state to another, we will catch it if we sum all available channels and thus retain response and underlying precision. As an added benefit, the summing also results in a smoothing of the resultant combined trace, facilitating peak definition and reproducible peak integration.
If you will humor me as I once again delve into my favorite domain of oligonucleotide quantification. It is a great working example of this topic, especially when we make a charge-state envelope-based comparison of the popular liquid chromatographic options that may be used, hydrophilic-interaction (HILIC) and reversed-phase (RP). We ought to consider what may be most analytically desirable as properties of our envelopes, for a quantitative endpoint, or ‘molar’ endpoint, you might say. In the important and growing domain of oligonucleotide bioanalysis, HILIC is now known to generate charge state distributions that, in comparison to ion-pairing reversed-phase, are narrower and show the predominantly intense charge state to be lower. A simpler situation. Less charge states in total to have to simultaneously look to bring the bat down on, and not so numerous in charges that effectively reduces peak spacing to a point where we wish we always had accurate mass at hand. Accurate mass makes it all easier with today’s technology. Also, with lower predominant charge states, we more readily avoid possibly bringing the m/z measurement too low for the most comfortable selectivity.
From experience, however, digging a little deeper may be called for, in a mole-like manner that could result in going against the proposed scheme and limiting the number of charge states monitored. This relates to the chance of encountering non-linearity and is something that my long whiskers touched in a real assay of late. It was a situation whereupon monitoring a decent number of charge states, we attained curvature of the ‘upward’ variety, where the peak area response began to take off exponentially with increasing nominal concentration. There was a little non-specific binding going on, but it was found that the spade-like feet behind it were to do with the charge states. We brought it down to only one being monitored, the most intense, and the problem was batted. To speculate a little, this is something that is almost certainly related to the dynamics of the production of gas-phase ions in the electrospray-based ion source; this may be alleviated or eliminated with micro- or nanoflow, where competition-based effects dwindle and can even practically disappear.
To sum up, if you will pardon the pun, I would advocate for capturing the visible charge states and their incorporation into an acquisition method. As such, any swings in abundance will be best accounted for, giving a more concrete concentration-response definition, plus it affords a beneficial smoothing effect. The aforementioned concrete is, of course, a mole’s enemy. However, if non-linearity is observed, it may be worth investigating a more select group or even a singular charge state to monitor.
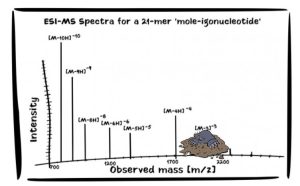
Image created by Hazel Dickson, Waters Corporation (MA, USA)
Disclaimer: the opinions expressed are solely my own and do not express the views or opinions of my employer.