The effects of sub-optimal sample handling on multiple complement measurements: Is there a potential signature of mishandling?
Ashley Frazer-Abel, Director at Exsera BioLabs (University of Colorado; CO, USA).
“To my work in complement immuno-biology, I brought my PhD in biochemistry and post docs in immunology. After initially working in clinical immunology, I expanded my focus to include work to support FDA IND enabling studies. At the time I started working in complement and non-clinical and clinical trials, most of the work we did was related to immuno-toxicology and the potential for adverse complement responses to the new biological classes of drugs. It is exciting to now be involved in testing for diagnosis and efficacy. My continued work in diagnostics has given me the opportunity to see patient lives change when a new drug is available for them and to know I played some part in getting that drug to market, safely.”

1. Please can you explain about your role at Exsera BioLabs?
I am the Laboratory Director of Exsera Biolabs, a specialized laboratory focusing on complement analysis for diagnostics, as well as non-clinical and clinical trials. As director I have a number of responsibilities but the most rewarding is the responsibility for determining which assays and tests need to be developed and validated next. This means we can take new discoveries and develop and validate a novel test today that will help provide answers for tomorrow, true translational medicine.
As complement is a small, but burgeoning field I am very involved in the international complement community. I work with the Complement Standardization and Quality Assessment Committee of the International Complement Society (ICS) which is also a subcommittee of the IUIS Quality Assessment and Standardization Committee. This ICS committee was officially formed in 2009 and is involved in number of efforts to improve complement analysis: from offering the most extensive internal quality assessment of complement analytes, to efforts to verify and publicize the best practices in specimen handling, to efforts to create a calibration standard for our myriad assays. I also hold a faculty appointment at the University of Colorado School of Medicine on the Anschutz Medical Campus (CO, USA) where I have the good fortune to work with a number of accomplished complementologists.
2. How does it feel to win the 12th WRIB Poster Award?
I was very honored and frankly surprised to win a WIRB Poster Award. While I believe in our work, complement is a relatively small field which led me to underestimate the interest. I am pleased to help raise awareness of the factors that can impact complement testing and what we can do to insure quality and reliable results.
3. Tell us a little about the work on which the poster was based?
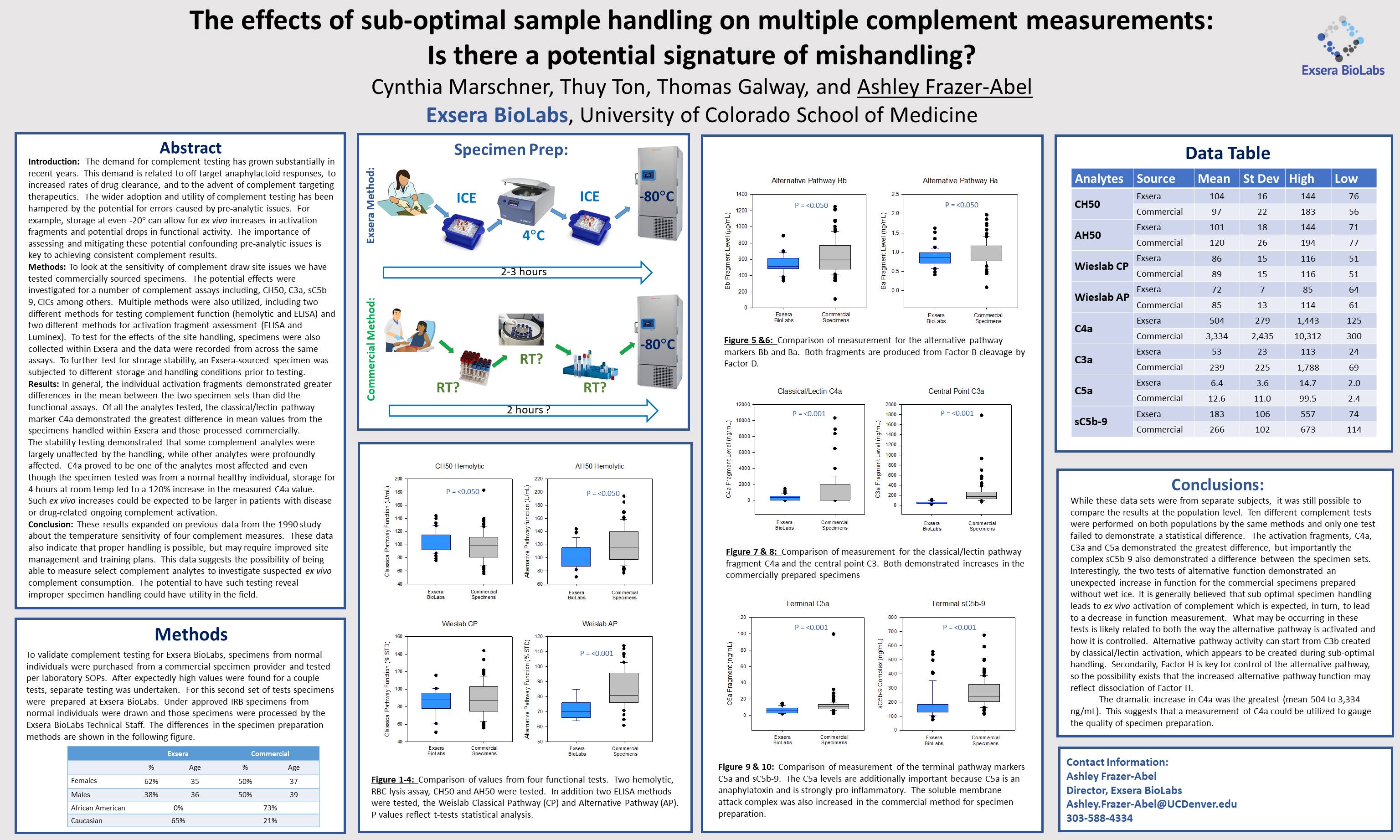
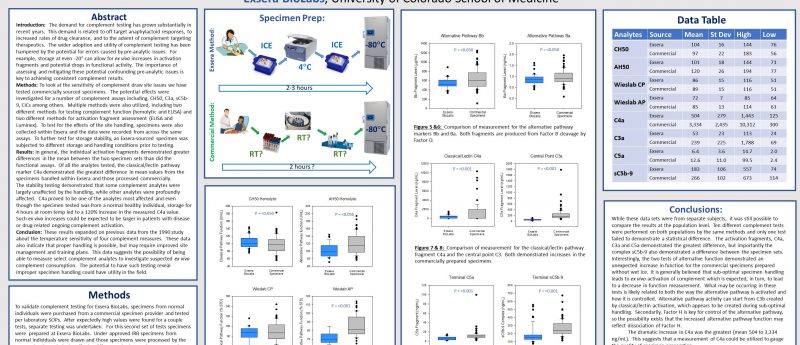
Experts in the field of complement have known for quite some time that complement is very sensitive to specimen handling. Complement is an enzymatic cascade, similar to coagulation, and is prone to ex vivo activation. Despite this historic knowledge there are very few publications that document the specific parameter and exact effects of mishandling on complement results; those that do exist do not generally reflect modern methods and the breadth of testing now available.
This sensitivity and lack of consensus has led some people to doubt complement results or discount their value. As we know the power and importance of complement, we initiated work to help clear-up the questions and doubts. Specifically, we started to look at the effects of specimen handling on our 30+ complement tests. In the work shown in the poster we focused on the potential effects of pre-analytic sample handling to determine whether it was necessary to prepare plasma and serum with ice and a refrigerated centrifuge. With the growing number of clinical trials that could benefit from quality complement testing, there is the real possibility that individual sites may default to room temperature handling, plus there is expense and effort in change. We tested our 30 analytes but on my poster I shared the data for the ten tests we most commonly handle. These ten included both functional measurements and level of activation markers, which are also two classes that can be the most sensitive to ex vivo activation. For the majority of the assays investigated, room temperature handling had a statistically significant effect on the analysis. Of those the C4a activation fragment demonstrated a large change with room temperature handling, which could have been expected. In addition there were other effects that were less expected, specifically the changes in alternative pathway function testing was counter to the expectation as it increases with room temperature handling. In addition there was a notable change in sC5b-9 which has been reported to be less sensitive.
4. What were the key conclusions from your research?
This work reconfirmed extends the understanding of the sensitivity of complement to suboptimal specimen handling. But importantly this work also demonstrates that it is possible to get more stable results by simply adding wet ice and refrigerated centrifugation to the process flow. And finally, if poor specimen handling seems likely that it may be possible to combine these results to find a ‘fingerprint’ of sub-optimal complement specimen handling.
5. What are you looking forward to working on over the next year?
In the coming year we will be working to extend this study by quantitating the effects of additional freeze/thaw cycles, as well as long and short term storage at different temperatures. When that quantification is completed we hope to publish this work. In addition, as part of my participation in the efforts of the Complement Standardization Committee, we plan to participate in publishing ICS approved recommendations for specimen handling. Beyond this work on quality, the next year promises to be busy for complement analysis, complement understanding and therapeutic drug development. It is an exciting time to be involved in this field.