Rapid generation of bispecific antibodies for high-throughput screening with SpyLock technology
In this interview with John Cardone (Bio-Rad; Oxford, UK), we explore bispecifics and their impact on the biologics landscape. John explains the use of SpyLock, a new approach to bispecific screening based on SpyTag technology, for the prototyping and early screening of bispecific antibodies.
 John Cardone
John Cardone
Marketing Manager, Custom Antibody Services
Bio-Rad
John Cardone is the Marketing Manager for Bio-Rad’s Custom Antibody Services including the newly developed Pioneer™ Antibody Discovery Platform and HuCAL® antibody library. Prior to this, he worked in an in-vitro immuno-diagnostic company as a Strategic Marketing Manager. John holds a PhD in Immunology from Kings College London and an MBA from Warwick Business School (both UK).
What are bispecifics and how are they impacting the biologics landscape?
Bispecific antibodies (BsAbs) are a class of biologics gaining significant interest in the field of biotherapeutics. So far, 13 bispecifics have been approved by regulatory agencies — FDA, EMA, and/or NMPA — across the globe and more than 450 are actively being investigated in clinical trials. Despite some interest in using BsAbs in clinical diagnostics and medical imaging, most bispecifics are used as cancer immunotherapies and to a lesser extent are being investigated for autoimmune or other diseases.
BsAbs exploit the biochemical properties of monoclonal antibodies (mAbs) but increase them two-fold with the presence of two engineered functional antibody domains that bind two different antigens, or two epitopes, on the same antigen [1] [see Fig. 1]. This allows the engagement of two targets through a single engineered bispecific molecule. BsAbs have several mechanisms of action (MOA). Most approved drugs act via bridging the effector and target cell (cell-bridging) to allow for the recruitment and activation of immune cells. The first BsAbs approved for use by the FDA exploit this MOA to treat B cell acute lymphoblastic leukemia. The targeted cancer biopharmaceutical blinatumomab comprises one arm that recognizes CD19 specific for B cells and a separate arm that recognizes T cells via CD3 engagement. This bispecific enables cancerous B cells to be targeted and killed via T cell-induced cytotoxicity [2].
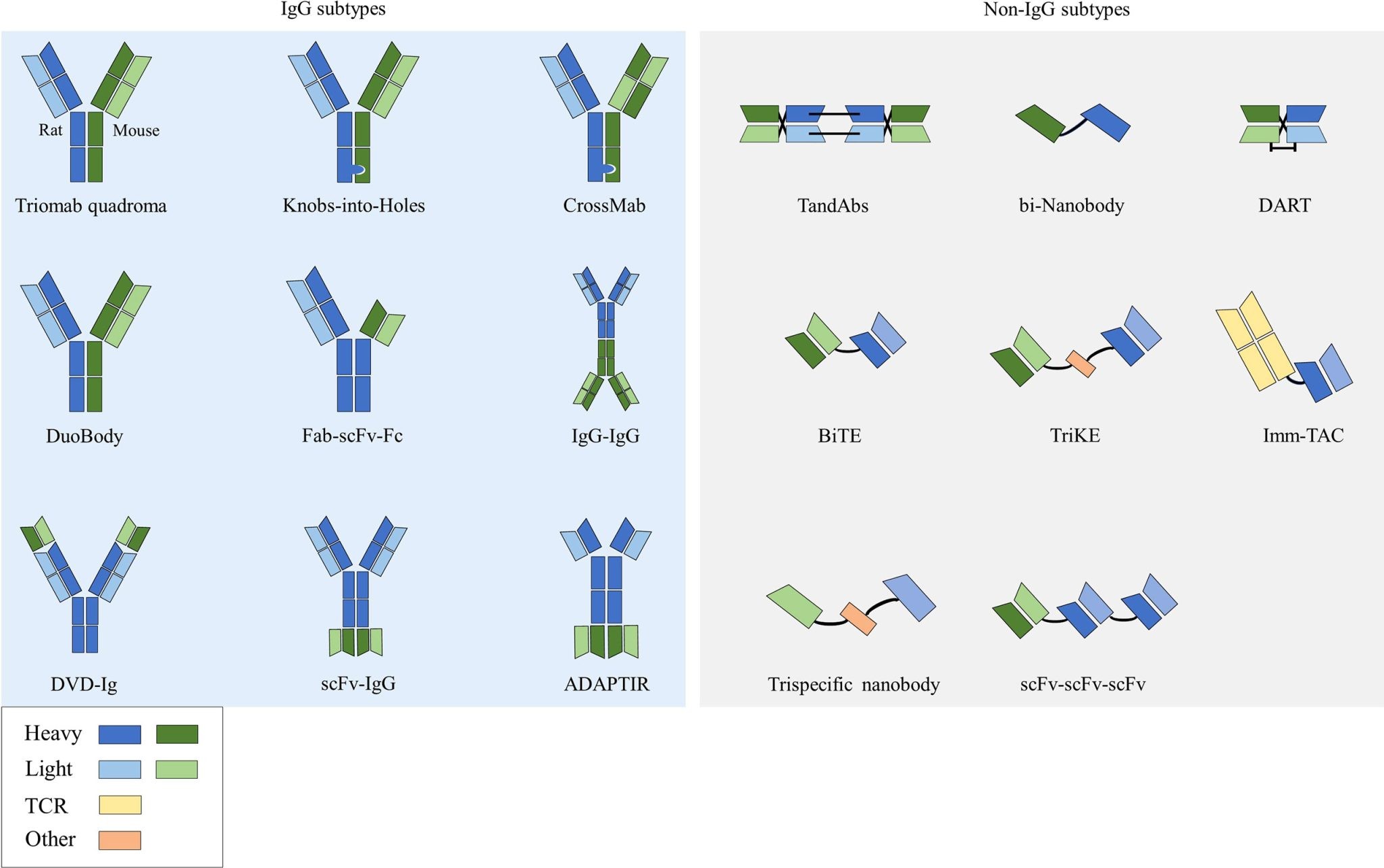
Fig 1. Structures and formats of bispecific antibodies [3].
Other MOAs comprise the dual targeting of receptor and ligand (to block immune checkpoints or inflammatory factors), the half-life extension of the compound and the homing of compounds facilitating delivery across membranes. Thanks to their MOA, bispecifics have the potential to further their impact on the biologics landscape in years to come. By compounding the properties of two mAbs within one molecule, patients may only need one treatment rather than combination therapies where multiple mAbs are administered, which present higher toxicity risks than single mAbs treatments. Moreover, the advantage of manufacturing a single molecule as opposed to two is also attractive to pharmaceutical companies. However, the trade-off is in the technical challenges that such ‘special’ antibodies pose versus mAbs, as well as the lack of ‘history’ concerning BsAbs.
Could you explain the key challenges in screening and bioanalysis of bispecifics?
The development of bispecifics presents challenges within all the steps of the discovery workflow, namely from discovery to pre-clinical and clinical phases to approval and commercialization. Although the first BsAbs approved by the EMA in 2009 were produced using hybridoma technology, it was soon clear that this approach had serious limitations from immunogenicity to manufacturability. Recombinant technologies are essential for the discovery and development of BsAbs, whether for the identification of new specific binding fragments or the humanization of existing candidates. Today, more than 30 platforms exist for the development of bispecific antibodies [1]. However, the work to screen for the relevant antibodies to build the bispecifics as well as their characterization process pose interesting challenges [4].
Currently, BsAbs can be classified as ‘IgG-like’ if they comprise an Fc region, or ‘non-IgG-like’ if they do not contain an Fc region. Therefore, depending on their format, different approaches need to be employed to develop PK and ADA assays. The standard process in BsAb discovery starts with a group of mAbs for the target antigens. The lead binders may be from already successful clones or identified de novo. Subsequently, BsAbs are produced via bioengineering and matching the antigen binding domains with different specificity through genetic fusion or antibody subunit heterodimerization. A limited number of candidates are usually then run through in-depth characterization assessments to ensure that the lead selection process focuses on both functional and developability parameters of hits. While this process of hit generation, selection, and characterization for mAbs usually involves looking at the properties of hundreds of single hits, bispecifics require considerations of hundreds or thousands of candidates, for example emicizumab — a clinically approved BsAb to treat hemophilia A — was identified after screening and optimizing 40,000 candidates. For instance, Wuellner et al. demonstrated that identical binders can differ in potency depending on the distance between the binders and that higher potency can be achieved with closer proximities of binders [5].
As for any biotherapeutic agent, bioanalytical methods are essential to inform on immunogenicity, PK and PD. To allow preclinical and clinical studies that will report on the safety and efficacy of the bispecific drugs, compound-specific antibody tools need to be generated. Beyond the different formats, bispecific antibodies add several layers of complexity to PK and PD studies mainly due to the presence of two targets rather than one, which can be present in different concentrations and on different cell types. As with mAbs, bispecific PK and ADA studies require antibodies that are inhibitory and noninhibitory to both the targets of interest.
Can you explain the key principles of generating the SpyLock technology?
Spylock technology is a new approach to bispecific screening based on SpyTag technology [2]. Briefly, SpyTag technology takes advantage of the autocatalytic reaction between SpyTag, a small peptide of 13 amino acids, and SpyCatcher, a small protein of 12.3 kDa, to form an isopeptide bond [4]. SpyTag and SpyCatcher are both derived from the fibronectin binding protein from Streptococcus pyogenes. The reaction is fast (minutes to an hour), specific and unaffected by low or high pH (5–8), different buffer conditions or the presence of detergents [6].
Using SpyLock, the Bio-Rad team has developed a ‘lockable’ catcher that can be reversibly inhibited to avoid the ligation with a SpyTag. The team has bioengineered the SpyLock catcher and incorporated it into a fusion protein alongside a standard catcher. The resulting construct, referred to as BiLockCatcher, features two sites enabling the loading of two antibodies. For example, antibody one can be loaded onto one site of BiLockCatcher while the second catcher (SpyLock) remains ‘locked’. Once the reaction of antibody one is complete, the SpyLock can be ‘unlocked’ using reducing reagents, allowing subsequent loading with antibody two. The process is simple and quick and is ideal for screening a large number of antibody combinations.
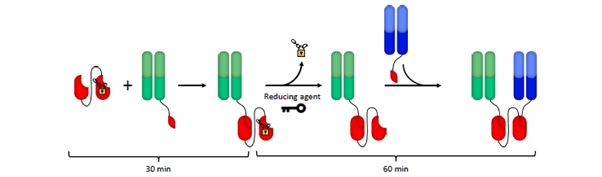
Fig 2. Workflow to produce bispecific antibodies with SpyLock and SpyTagged Fabs. SpyLock technology can be used to generate bispecific antibodies very rapidly. A SpyCatcher-SpyLock dimer can be reacted with a first antibody, followed by unlocking with reducing agent and simultaneous reaction with a second antibody. Bio-Rad have developed a protocol that can be performed in 90 minutes, as well as a high purity protocol, which includes purification steps and usually yields >95% bispecific antibody. [2]
How can SpyLock be used for the early screening of bispecific antibodies specifically?
A straightforward application of SpyLock is the generation of bispecific antibodies. What all bispecific antibodies have in common is that the screening space grows quadratically with the number of antibody sequences to be tested in combination. Given that the production of final therapeutic BsAb formats is typically labor-intensive, minimizing the number of BsAbs needing conversion into the final format is valuable. Therefore, implementing a preliminary screening step in an alternative format to assess general functionality can massively reduce workload and accelerate candidate selection.
References:
- Ma J, Mo Y, Tang M et al. Bispecific antibodies: from research to clinical application. Front. Immunol. 12, 626616 (2021).
- Hentrich C, Putyrski M, Hanuschka H et al. Engineered reversible inhibition of SpyCatcher reactivity enables rapid generation of bispecific antibodies. doi:1101/2023.10.27.564397 (2023) (Epub ahead of print).
- Kang J, Sun T, Zhang Y. Immunotherapeutic progress and application of bispecific antibody in cancer. Front. Immunol. 13, 1020003 (2022).
- Segaliny AI, Jayaraman J, Chen X et al. A high throughput bispecific antibody discovery pipeline. Commun. Biol. 6(1), 380 (2023).
- Wuellner U, Klupsch K, Buller F et al. Bispecific CD3/HER2 targeting FynomAb induces redirected T cell-mediated cytolysis with high potency and enhanced tumor selectivity. Antibodies. 4, 426-440 (2015).
- Hentrich C, Kellmann S-J, Putyrski M et al. Periplasmic expression of SpyTagged antibody fragments enables rapid modular antibody assembly. Cell Chem. Biol. 28(6), 813–824 (2021).
Further reading:
- Beacon bispecific antibodies and immune checkpoint modulators: a H1 landscape analysis. (2023) https://beacon-intelligence.com/infographic/global-commercial-insights-from-the-bispecific-market/ [Accessed 24 January 2024].
- Bio-Rad. SpyTag and SpyCatcher products. (2023): www.bio-rad-antibodies.com/spytag-spycatcher-products.html [Accessed 24 January 2024].
In association with: